��Ŀ����
4���±�ΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã��û�ѧ����ش���������| �� ���� | ��A | 0 | ||||||
| 1 | �� | II A | IIIA | IVA | VA | VIA | V��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����HNO3��H2CO3��H2SiO3��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��
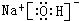 ��
�� �ȣ�
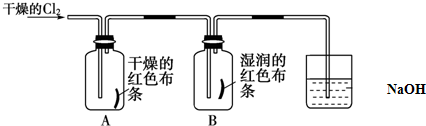
�ȣ���4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������A��ϡ��Һ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�
ab��a��MnO2������ b��FeCl3������ c��Na2SO3������ d��KMnO4
��5�����з�̪��NaOHϡ��Һ�У���ε���10%������A��Һ����ɫ�ܿ���ȥ����ɫԭ��ͬѧ��ΪA��Һ���������£���ͬѧ��ΪA��Һǿ���������£��������һ����ʵ����˵���Ի����Ҷԣ�
����������˵��������ɫ�����Һ������ε���NaOH��Һ������ɫ���ָ���˵���Ҷԣ�
���� ����Ԫ�������ڱ��е�λ��֪��Ԫ�آ١���ֱ���H��C��N��O��Na��Al��Si��ClԪ�أ�
��1��ԭ�ӵ��Ӳ���Խ��ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С��
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ��
��3�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������A��ϡ��Һ�ױ����ֽ⣬������Ϊ˫��ˮ���������̡��Ȼ���������˫��ˮ�ֽ�Ĵ�����
��5����������ɫ��Һ�м�������������Һ��������������жϣ�
��� �⣺����Ԫ�������ڱ��е�λ��֪��Ԫ�آ١���ֱ���H��C��N��O��Na��Al��Si��ClԪ�أ�
��1��ԭ�ӵ��Ӳ���Խ��ԭ�Ӱ뾶Խ��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ�������������С������������Ԫ��ԭ�Ӱ뾶��С˳����Na��Al��O���ʴ�Ϊ��Na��Al��O��
��2��Ԫ�صķǽ�����Խǿ��������������ˮ��������Խǿ���ǽ�����N��C��Si��������������ˮ��������ǿ��˳����HNO3��H2CO3��H2SiO3���ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3�����ý����ͻ��÷ǽ���Ԫ��֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ����⼸��Ԫ���еļ������γɺ������Ӽ��ͼ��Լ��Ļ��������ʽΪ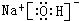 ��
�� �ȣ��ʴ�Ϊ��
�ȣ��ʴ�Ϊ��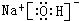 ��
�� �ȣ�
�ȣ�
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬������A��ϡ��Һ�ױ����ֽ⣬������Ϊ˫��ˮ���������̡��Ȼ���������˫��ˮ�ֽ�Ĵ�������ѡab��
��5������ɫ����Һ�еμ�����������Һ������ɫ�ָ���˵��˫��ˮ�����Ե��µ���Һ��ɫ������ɫ���ָ���˵��˫��ˮ�н�ǿ�����ԣ�����̪�����ˣ�
�ʴ�Ϊ������ɫ��Һ������μ���NaOH��Һ������ɫ�ָ�˵���ԣ�����ɫ���ֵ���˵���Ҷԣ�
���� ���⿼��Ԫ�����ڱ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�漰�������ʼ��顢���ʽṹ��Ԫ�������ɵ�֪ʶ�㣬��ȷ���ʽṹ�����ʹ�ϵ�ǽⱾ��ؼ����ѵ��ǣ�5������

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�| A�� |  �ų���ı���Һ | B�� |  ����Ⲣ���ձ� | ||
| C�� |  ���������ճɻ� | D�� |  ���˵ĺ�I-��Һ |
�ٱ���Na2SO3��Һ ������KMnO4��Һ ��ʯ��ˮ ����ˮCuSO4 ��Ʒ����Һ��
| A�� | �ܢݢ٢ڢ� | B�� | �ܢݢ٢ۢ� | C�� | �ݢ٢ۢڢ� | D�� | �ܢۢ٢ݢ� |
��������
| A�� | 120.4g/mol | B�� | 50.4g/mol | C�� | 182.4g/mol | D�� | 252g/mol |
 ��
��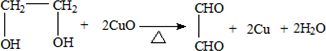 ��
�� ��
��