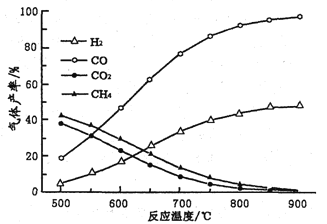
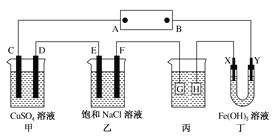
��Ŀ����
����Ŀ����1����������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
��ͼ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2������������ClO2�ĵ缫��ӦʽΪ___________________________________________________________________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ________mol����ƽ���ƶ�ԭ������������pH�����ԭ��___________________________________________________________
��2��Ϊ��״�ȼ�ϵ������ʣ���ѧ�ҷ�����һ��ȼ�ϵ�أ���ص�һ���缫ͨ���������һ���缫ͨ��״����壬������Dz�����Y2O3��ZrO2���壬�ڸ��������ܴ���O2������ع���ʱ������ӦʽΪ___________________
���𰸡�Cl��- 5e����2H2O=ClO2����4H�� 0.01 ����������2H����2e��=H2����H��Ũ�ȼ�С��ʹH2O![]() H����OH����ƽ�������ƶ���OH��Ũ������pH���� O2��4e��=2O2��
H����OH����ƽ�������ƶ���OH��Ũ������pH���� O2��4e��=2O2��
��������
(1) ����������������Ӧ���������֪�������ӷŵ�����ClO2����Ԫ���غ��֪����ˮ�μӷ�Ӧ����ϵ���غ��֪��ͬʱ���������ӣ�
��������������Ӧ��2H++2e-=H2��������n=V/Vm�������������ʵ�����ͨ�������ӽ���Ĥ��������Ϊ+1�����ӣ����ݵ���ת���غ���������ӵ����ʵ����������H+Ũ�ȼ�С��ʹ��H2O![]() OH-+H+��ƽ�������ƶ�;
OH-+H+��ƽ�������ƶ�;
(2) �������ʵ�ȼ�ϵ�أ�ͨ������ĵ缫Ϊ�������״������ڸ�������������Ӧ��
��1������������������Ӧ���������֪�������ӷŵ�����ClO2����Ԫ���غ��֪����ˮ�μӷ�Ӧ����ϵ���غ��֪��ͬʱ���������ӣ������缫��ӦʽΪ��Cl--5e-+2H2O=ClO2��+4H+���ʴ�Ϊ��Cl--5e-+2H2O=ClO2��+4H+��
������������2H++2e-=H2�������������ʵ���Ϊ0.112L��22.4L/mol=0.005mol��ͨ�������ӽ���Ĥ��������Ϊ+1�����ӣ��ʽ���Ĥ�������ӵ����ʵ���Ϊ0.005mol��2=0.01mol�����������H+Ũ�ȼ�С��ʹ��H2OOH-+H+��ƽ�������ƶ�����Һ��pH���ʴ�Ϊ��0.01������������2H++2e-=H2����H+Ũ�ȼ�С��ʹ��H2O![]() OH-+H+��ƽ�������ƶ���OH-Ũ������pH������
OH-+H+��ƽ�������ƶ���OH-Ũ������pH������
��2��ȼ�ϵ���У�һ���缫ͨ���������һ���缫ͨ��״����壬������Dz����� Y2O3��ZrO2���壬�ڸ��������ܴ���O2-���ӣ�����O2�õ���������O2-��O2+4e-=2O2-���ʴ�Ϊ��O2+4e-=2O2-��