��Ŀ����
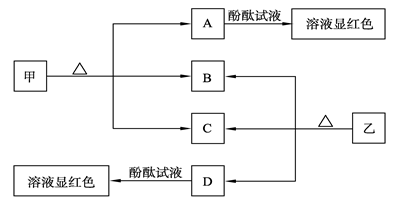
�ǽ�������A��������ͼ��ʾ�Ĺ���ת��Ϊ������D����֪DΪ����ǿ��

��ش��������⣺
��1����A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ����
��D�Ļ�ѧʽ��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ��� ����Ⱦ�˻���
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣��A��C�Ļ�ѧʽ�ֱ��ǣ�A ��C
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ

��ش��������⣺
��1����A�ڳ�����Ϊ���壬B����ʹƷ����Һ��ɫ���д̼�����ζ����ɫ����
��D�Ļ�ѧʽ��
���ڹ�ҵ������B����Ĵ����ŷű���ˮ���պ��γ��� ����Ⱦ�˻���
��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ���塣��A��C�Ļ�ѧʽ�ֱ��ǣ�A ��C
��D��Ũ��Һ�ڳ����¿���ͭ��Ӧ������C���壬��д���÷�Ӧ�Ļ�ѧ����ʽ
��1����H2SO4 ������
��2����N2 NO2 ��Cu��4HNO3(Ũ)=Cu(NO3)2��2H2O��2NO2
��2����N2 NO2 ��Cu��4HNO3(Ũ)=Cu(NO3)2��2H2O��2NO2
�������������Ԫ�ػ�����֪ʶ�������ǽ������ʿ���ʵ������ת���ķֱ���C��S��N2�� DΪǿ�ᣬ����̼���ʵ�ת������1����B����ʹƷ����Һ��ɫ�ı��д̼�����ζ����ɫ���壬BΪSO2�����Ծݴ��ƶ�CΪSO3��DΪH2SO4����SO2����ˮ���պ�����H2SO3��H2SO4�����γ����ꣻ��2����A�ڳ�����Ϊ���壬C�Ǻ���ɫ����ΪNO2��AΪN2���ھݢٿ�֪DΪ���ᣬŨ������ͭ��Ӧ����NO2����Ϊ��Cu+4HNO3��Ũ��=Cu��NO3��2+2H2O+2NO2����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ