��Ŀ����
����Ŀ��Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
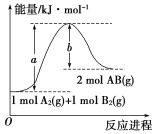
(1)��֪��ѧ��ӦA2(g)��B2(g)===2AB(g)�������仯��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ____��
(2)ʵ���ã�1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ����������д���Ҵ�ȼ�յ��Ȼ�ѧ����ʽ��_______��
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)===CH3COOH(l)���ʱ�Ϊ_________��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)===CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)===H2O(l) ��H3����285.8 kJ��mol��1
O2(g)===H2O(l) ��H3����285.8 kJ��mol��1
���𰸡�A2(g)��B2(g)===2AB(g)����H��(a��b) kJ��mol��1 C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)����H����1366.2 kJ��mol��1 ��488.3 kJ��mol��1
��������
��1��������H=��Ӧ��Ļ��-�����������㣻
��2��1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ�����������1mol�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7��46 kJ=1366.2 kJ��������д���Ȼ�ѧ����ʽ��
��3����CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
��ϸ�˹���ɿ�֪���ڡ�2+�ۡ�2-���õ�2C��s��+2H2��g��+O2��g���TCH3COOH��l�����Դ������
��1����ͼ������H=��Ӧ����-��������=��a-b��kJ��mol��1 ����Ӧ���Ȼ�ѧ����ʽ��A2(g)��B2(g)=2AB(g)����H��(a��b) kJ��mol��1��
��2��1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ�����������1mol�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7��46 kJ=1366.2 kJ��������д���Ȼ�ѧ����ʽ��C2H5OH(l)��3O2(g)=2CO2(g)��3H2O(l)����H����1366.2 kJ��mol��1 ��
��3����CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
��ϸ�˹���ɿ�֪���ڡ�2+�ۡ�2-���õ�2C��s��+2H2��g��+O2��g���TCH3COOH��l��������H=��-393.5kJ��mol��1����2+��-285.8kJ��mol��1����2-��-870.3kJ��mol��1��=-488.3kJ��mol��1��

����Ŀ�������������ж��֣��������ȿ���������ˮ������84����Һ���ڼ�ͥ�����ݵ�������
I.ʵ���ҿ����������ƹ�����Ӧ�Ʊ�ClO2��2NaClO2+Cl2=2ClO2+2NaCl��װ����ͼ��ʾ��
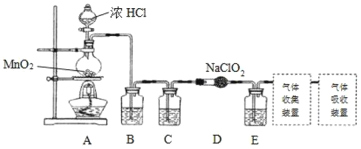
��1��װ��A�У�ʢװŨ�������������Ϊ___����ʼ��Ӧ��Բ����ƿ�ڷ����ķ�Ӧ�����ӷ�Ӧ����ʽΪ��__��
��2����֪���³�ѹ�£�ClO2��Cl2�����壬�ڲ�ͬ�ܼ����ܽ��������ʾ��B��C��Eװ���е��Լ�������___�����ţ�
ClO2 | Cl2 | |
ˮ | ��������ˮ | �� |
CCl4 | ���� | ���� |
a.Ũ���� b.����ʳ��ˮ c.NaOH��Һ d.CCl4
��3��ʹ��ClO2�ڸ�����ˮ�����Ĺ����л�����к��ĸ��������������ClO2-��������Fe2+����ȥ������֪ClO2-��Fe2+��pH=5��7���������ܿ��ٷ�Ӧ�������γɺ��ɫ��������ClO2-��ԭ��Cl-��Fe2+����ClO2-�����ӷ���ʽΪ__��
II.ijͬѧ�ڼ������Ƴ�����84����Һ���ɷ֣�NaClO��ˮ����ͬ������Һ����Ҫ������6V��ѹ������֧ľ��Ǧ�ʡ��ϴ���ˮƿ��ʳ�Ρ�����ֽ����Ե������С���ȡ�
��4��ʵ����̣���һ�������Ĵ���ˮƿ�й�����ƿ����ˮ��������3��ζ��ʳ�Σ�������ֽ����Ǧ�����ɵĵ缫���ã������봿��ˮƿ�У�ʹ�缫ǡ�ÿ���ƿ�ڣ�װ����ͼ����ͨ��Դ���Կ���һ��缫������������һ��缫��ϸС�����ݲ�������д���õ缫��ӦʽΪ��___������ͨ��Լ3Сʱ����ԭ������������ĵ缫����Ҳ��ʼ����һ������ϸС���ݣ��˵缫��ʱ�ĵ缫��Ӧʽ___�������������ֹͣͨ�硣

��5���ø÷����Ʊ�����Һ���ܻ�ѧ����ʽ�ǣ�___��