��Ŀ����
7�� ij��ѧ��ȤС��ͬѧ���ú�°��Ƽ��ԭ���Ƶ���һЩ��������飬��Ʒ�к�������NH4Cl��������ʵ��ⶨ��Ʒ�Ĵ��ȣ�����˶���̽��������
ij��ѧ��ȤС��ͬѧ���ú�°��Ƽ��ԭ���Ƶ���һЩ��������飬��Ʒ�к�������NH4Cl��������ʵ��ⶨ��Ʒ�Ĵ��ȣ�����˶���̽������������һ���������ⶨ��Ʒ��Cl-����������������Ʒ�м����ữ����������Һ����������AgCl��������
���������������ⶨ��Ʒ��CO32-����������������Ʒ�м�������BaCl2��Һ����CO32-�������������BaCO3��������
����������ϡ�ὫCO32-ת����CO2���������ռ�����CO2��ͨ���ⶨ���ռ����������㴿�ȣ�
�Իش��������⣺
��1������һ��Ӧѡ����������ữ������һ�������辭����ȡ�����������ڵμ���Һ���۹��ˣ���ϴ�ӣ��ݸ�������������㶨��
��2����ͬѧѡ���õ����ַ����ⶨ��Ʒ���ȣ����������ͼװ�ã�
��װ��C�������Ƿ�ֹ�����е�CO2��ˮ������B�м�ʯ�����գ�
�ڴ�װ��A��ർ�ܴ�ͨ������Ŀ����ʹ�����ɵ�CO2����ȫ����B�м�ʯ�����գ�Ϊ��������ʵ��������Ϊ��װ�û�Ӧ�����θĽ�����װ��A����һ��װ�ã��Գ�ȥ����װ��A�Ŀ����е�CO2��
��3����ͬѧָ���������ñ�����ζ���Ʒ��Һ��Na2CO3����ȫת��ΪNaCl�ķ����ⶨ���ȣ�����Ϊ�÷�����Ҫ֪����ⶨ�����ݣ���ͬʱ����ĸ��ʾ����ע���������λ��������Ʒ����m g������Ҫ�������Ũ��c mol•L-1�������������V mL�������������ݼ�����Ʒ��Na2CO3����������$\frac{cV��1{0}^{-3}106}{2m}$��
���� ��1������AgCl��������Ҫ���������ữ����������Һ����������ʱ����Ҫ��ϴ�ӡ����
��2����װ��C���տ����е�CO2��ˮ��������ֹB������
�ڴ�װ��A��ർ�ܴ�ͨ�������ϳ�CO2��ʹ�����ɵ�CO2����ȫ����B�м�ʯ�����գ�Ϊ��������ʵ����Ӧ��װ��A����һ��װ�ã��Գ�ȥ����װ��A�Ŀ����е�CO2��
��3���ñ�����ζ���Ʒ��Һ��Na2CO3����ȫת��ΪNaCl�ķ����ⶨ���ȣ���Ҫ֪���������Ũ�Ⱥ���������ݹ�ϵʽ������Na2CO3������������
��� �⣺��1������AgCl��������Ҫ���������ữ����������Һ����������ʱ����Ҫ��ϴ�ӡ����
�ʴ�Ϊ�����ϴ�ӣ����
��2����װ��C���տ����е�CO2��ˮ��������ֹB�������ʴ�Ϊ����ֹ�����е�CO2��ˮ������B�м�ʯ�����գ�
�ڴ�װ��A��ർ�ܴ�ͨ�������ϳ�CO2��ʹ�����ɵ�CO2����ȫ����B�м�ʯ�����գ�Ϊ��������ʵ����Ӧ��װ��A����һ��װ�ã��Գ�ȥ����װ��A�Ŀ����е�CO2��
�ʴ�Ϊ��ʹ�����ɵ�CO2����ȫ����B�м�ʯ�����գ���װ��A����һ��װ�ã��Գ�ȥ����װ��A�Ŀ����е�CO2��
��3���ñ�����ζ���Ʒ��Һ��Na2CO3����ȫת��ΪNaCl�ķ����ⶨ���ȣ���Ҫ֪���������Ũ�Ⱥ������
���ݹ�ϵʽ����Na2CO3��2HCl
1 2
n��Na2CO3�� cV��10-3mol��n��Na2CO3��=$\frac{cV��1{0}^{-3}}{2}$mol��m��Na2CO3��=nM=$\frac{cV��1{0}^{-3}��106}{2}$g��
����Ʒ��Na2CO3����������Ϊ$\frac{cV��1{0}^{-3}106}{2m}$��
�ʴ�Ϊ���������Ũ��c mol•L-1�������������V mL��$\frac{cV��1{0}^{-3}106}{2m}$��
���� ���⿼��ʵ���Ҳⶨ����Ĵ��ȣ��Ѷ��еȣ�ע���ų������е�CO2��ˮ������ʵ���Ӱ�죬��ϵʽ���ļ��㣮

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 0.1mol•L-1CH3COONa��Һ�У�H+��Ca2+��Cl-��NO3- | |
| B�� | ������Һ�У�Fe3+��Na+��Br-��SO42- | |
| C�� | �ں��б��ӵ���Һ�У�K+��NH4+��Br-��Fe3+ | |
| D�� | ����������Һ�У�K+��Al3+��SO42-��MnO4- |
| A�� | ����H20�����е����������ڷ�Ӧ��H2��O2�����е������� | |
| B�� | ��Ӧ��H2�����е��������ڲ���H2O�����е������� | |
| C�� | ��Ӧ��H2��O2�����е����������ڲ���H2O�����е������� | |
| D�� | ��Ӧ��H2��02���е�������� |
| A�� | Cu��Al | B�� | Mg��Al | C�� | Al�� Zn | D�� | Mg �� Zn |
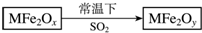 �������ײ���MFe2Ox��3��x��4����M��ʾ+2�۵Ľ���Ԫ�أ��ڷ�Ӧ�л��ϼ۲������仯�������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS��������ͼ���������ж���ȷ���ǣ�������
�������ײ���MFe2Ox��3��x��4����M��ʾ+2�۵Ľ���Ԫ�أ��ڷ�Ӧ�л��ϼ۲������仯�������£�MFe2Ox��ʹ��ҵ�����е�SO2ת��ΪS��������ͼ���������ж���ȷ���ǣ�������| A�� | MFe2Ox�������� | B�� | SO2�Ǹ÷�Ӧ�Ĵ��� | ||
| C�� | x��y | D�� | MFe2Oy�ǻ�ԭ���� |

| A�� | a��b������ʱ����Ƭ�ϻ��н���ͭ�������Ӷ�ʹ���ĸ�ʴ���ʼӿ� | |
| B�� | a��b�õ�������ʱ��ͭƬ�Ϸ����ķ�ӦΪCu2++2e-�TCu | |
| C�� | a��b�ֱ�����ֱ����Դ������������ʵ�����϶�ͭ����ʹCu2+��ͭ�缫�ƶ� | |
| D�� | ����a��b�Ƿ����ӣ���Ƭ�����ܽ� |
| A�� | ����ʹ��ú�������γ���������Ҫԭ��֮һ | |
| B�� | �ԡ��ع��͡�������Ի������ | |
| C�� | �ԷϾɵ�ؽ��л��մ�����ҪΪ�˷�ֹ�ؽ�����ȾˮԴ | |
| D�� | �뵼����ҵ����һ�仰������ɳ̲���û����������оƬ�IJ����ǹ� |
| A�� | ��YԪ���γɵĵ��������壬��XԪ���γɵĵ���һ��Ҳ������ | |
| B�� | ��HnXOmΪǿ�ᣬ��X���⻯����Һ��ˮһ�������� | |
| C�� | ��X��Yԭ���������1��YΪ��A�壬��Xһ���Ǣ�A�� | |
| D�� | ��Y��OH��m������ˮ����X��OH��nһ��������ˮ |