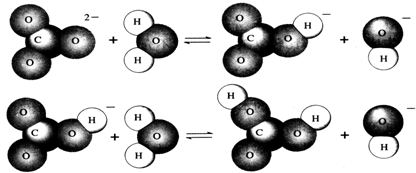
��Ŀ����
��1����������Һ���ܷ���ˮ��������ӷ���ʽ��ʾ�����ܷ���ˮ�����д�ϡ�������ˮ�⡱��������˵����Һ������ԣ�
K2CO3______����Һ��______�ԣ�
K2SO4______����Һ��______�ԣ�
NH4Cl______����Һ��______�ԣ�
��2�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��������Һ����KNO3����Na2CO3����NaHCO3����NaHSO4����CH3COOH����NaOH����Ba��OH��2����NH4Cl��pH�ɴ�С��˳��Ϊ��______�������ִ��ţ�
��3�����г����µ�0.1mol?L-1Na2CO3��Һ��
������Ϊ����Һ�ʼ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ��______��
��Ϊ֤����������۵㣬�����һ����ʵ�飬����ʵ�����______��
K2CO3______����Һ��______�ԣ�
K2SO4______����Һ��______�ԣ�
NH4Cl______����Һ��______�ԣ�
��2�����ʵ���Ũ�Ⱦ�Ϊ0.1mol/L��������Һ����KNO3����Na2CO3����NaHCO3����NaHSO4����CH3COOH����NaOH����Ba��OH��2����NH4Cl��pH�ɴ�С��˳��Ϊ��______�������ִ��ţ�
��3�����г����µ�0.1mol?L-1Na2CO3��Һ��
������Ϊ����Һ�ʼ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ��______��
��Ϊ֤����������۵㣬�����һ����ʵ�飬����ʵ�����______��
��1��K2CO3��ǿ�������Σ�ˮ���ˮ��Һ���ʼ��ԣ�ˮ�����ӷ���ʽΪCO32-+H2O?HCO3-+OH-��
Na2SO4��ǿ��ǿ���Σ���ˮ�⣬��Һ�����ԣ�
NH4Cl��ǿ�������Σ�ˮ�����Һ�����ԣ�ˮ�����ӷ���ʽΪNH4++H2O?NH3?H2O+H+��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-�����ˮ�⣻���ԣ�NH4++H2O?NH3?H2O+H+���
��2�����CH3COOH��������ʣ�����ֻ�в��ֵ��룬��c��H+����0.1mol/L������pH��1��
���NaOH��ǿ����ʣ���ȫ���룬c��OH-��=0.1mol/L������pH=13��
��Ba��OH��2��ǿ����ʣ���ȫ���룬c��OH-��=0.2mol/L������pH=13.7��
�Σ���NaHSO4��ǿ����ʽ�Σ���ˮ����ȫ����������ӡ���������ӡ������ӣ�����c��H+��=0.1mol/L������pH=1��
��NH4Cl��ǿ�������Σ�ˮ�����Һ�����ԣ�1��pHֵ��7��
��KNO3��ǿ��ǿ���Σ�ˮ��Һ�����ԣ�pH=7��
��Na2CO3��ǿ�������Σ�ˮ��Һ���ʼ��ԣ�
��NaHCO3���������ʽ��ˮ���Լ��ԣ�ˮ��̶�С��Na2CO3 ������С��Na2CO3������pH�ɴ�С��˳��Ϊ���ߣ��ޣ��ڣ��ۣ��٣��ࣾ�ݣ��ܣ�
�ʴ�Ϊ���ߣ��ޣ��ڣ��ۣ��٣��ࣾ�ݣ��ܣ�
��3����Na2CO3��ǿ�������Σ�ˮ���ˮ��Һ���ʼ��ԣ���ˮ�����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
����Na2CO3��Һ�мӷ�̪��Һ����Һ���ɫ��˵����Һ�Լ��ԣ�֤��Na2CO3ˮ�⣬
�ʴ�Ϊ����Na2CO3��Һ�мӷ�̪��Һ����Һ���ɫ��˵����Һ�Լ��ԣ�֤��Na2CO3ˮ�⣮
Na2SO4��ǿ��ǿ���Σ���ˮ�⣬��Һ�����ԣ�
NH4Cl��ǿ�������Σ�ˮ�����Һ�����ԣ�ˮ�����ӷ���ʽΪNH4++H2O?NH3?H2O+H+��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-�����ˮ�⣻���ԣ�NH4++H2O?NH3?H2O+H+���
��2�����CH3COOH��������ʣ�����ֻ�в��ֵ��룬��c��H+����0.1mol/L������pH��1��
���NaOH��ǿ����ʣ���ȫ���룬c��OH-��=0.1mol/L������pH=13��
��Ba��OH��2��ǿ����ʣ���ȫ���룬c��OH-��=0.2mol/L������pH=13.7��
�Σ���NaHSO4��ǿ����ʽ�Σ���ˮ����ȫ����������ӡ���������ӡ������ӣ�����c��H+��=0.1mol/L������pH=1��
��NH4Cl��ǿ�������Σ�ˮ�����Һ�����ԣ�1��pHֵ��7��
��KNO3��ǿ��ǿ���Σ�ˮ��Һ�����ԣ�pH=7��
��Na2CO3��ǿ�������Σ�ˮ��Һ���ʼ��ԣ�
��NaHCO3���������ʽ��ˮ���Լ��ԣ�ˮ��̶�С��Na2CO3 ������С��Na2CO3������pH�ɴ�С��˳��Ϊ���ߣ��ޣ��ڣ��ۣ��٣��ࣾ�ݣ��ܣ�
�ʴ�Ϊ���ߣ��ޣ��ڣ��ۣ��٣��ࣾ�ݣ��ܣ�
��3����Na2CO3��ǿ�������Σ�ˮ���ˮ��Һ���ʼ��ԣ���ˮ�����ӷ���ʽΪ��CO32-+H2O?HCO3-+OH-��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��
����Na2CO3��Һ�мӷ�̪��Һ����Һ���ɫ��˵����Һ�Լ��ԣ�֤��Na2CO3ˮ�⣬
�ʴ�Ϊ����Na2CO3��Һ�мӷ�̪��Һ����Һ���ɫ��˵����Һ�Լ��ԣ�֤��Na2CO3ˮ�⣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 +Br2
+Br2 +HBr
+HBr