��Ŀ����
����Ŀ���ش��������⣺
��1����̬Gaԭ�Ӽ۵����Ų�ͼΪ________��
��2�����ⶨ���֣�N2O5������NO2+��NO3-����������ɣ��ù�����Nԭ���ӻ�����Ϊ___;��NO2+��Ϊ�ȵ����������_____(д��һ��)��
��3���縺��:��_____��(����>������<��)����һ�������״������ԭ����________��
��4��NH3�����ڶ�������ʱH��N��H����Ϊ106.7������ͼ[Zn(NH3)6]2�����ӵIJ��ֽṹ�Լ�H��N��H���ǵIJ���ֵ�������������H��N��H���DZ�Ϊ109.5����ԭ��_______________________________��

��5����֪ͼ����������Ϊ[PtCl6]2-���ӣ�����ΪK+�������徧���߳�Ϊa pm��K2PtCl6�����ʽ��ΪM�������ӵ�������ֵΪNA�����ܶ�Ϊ________g��cm3���г�����ʽ���ɣ���

���𰸡�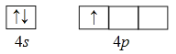 sp��sp2 SCN-��CO2��CS2��N3-���е���һ�� < ��ԭ��3p������ڰ����״̬��������ʧȥ���� ��������Zn2���γ������¶Ե�����Zn2���ɼ���ԭ�¶Ե�������Ե��Ӽ���ų����ñ�Ϊ���Ե��Ӽ���ų⣬�ų��������H��N��H���DZ�� 4��1030M/(a3 NA)
sp��sp2 SCN-��CO2��CS2��N3-���е���һ�� < ��ԭ��3p������ڰ����״̬��������ʧȥ���� ��������Zn2���γ������¶Ե�����Zn2���ɼ���ԭ�¶Ե�������Ե��Ӽ���ų����ñ�Ϊ���Ե��Ӽ���ų⣬�ų��������H��N��H���DZ�� 4��1030M/(a3 NA)
��������
��1����̬Gaԭ�Ӽ۵���4s24p1���ɴ�д���۵����Ų�ͼ ��
��
��2��NO2����Nԭ�Ӽ۲���ӶԸ���=2+��5-1-2��2��/2=2��NO3����Nԭ�Ӽ۲���ӶԸ���=3+(5+1-2��3)/2=3�����ݼ۲���ӶԻ��������ж�Nԭ���ӻ����ͣ���NO2����Ϊ�ȵ���������к���3��ԭ�ӡ��۵�������16��
��3��ͬһ����Ԫ�أ�Ԫ�صĵ縺������ԭ����������Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��4����������Zn2���γ������¶Ե�����Zn2���ɼ���ԭ�¶Ե�������Ե��Ӽ���ų����ñ�Ϊ���Ե��Ӽ���ų⣬�ų��������H��N��H���DZ��
��5���ɾ�̯������Ϧ�=m/V���㡣
��1����̬Gaԭ�Ӽ۵���4s24p1���ɴ�д���۵����Ų�ͼ�� ��
��
��2��NO2����Nԭ�Ӽ۲���ӶԸ���=2+��5-1-2��2��/2=2��NO3����Nԭ�Ӽ۲���ӶԸ���=3+(5+1-2��3)/2=3�����ݼ۲���ӶԻ��������ж�Nԭ���ӻ����ͣ�ǰ����sp�ӻ���������sp2����NO2����Ϊ�ȵ���������к���3��ԭ�ӡ��۵�������16��������ӻ�Ϊ�ȵ��������SCN����CO2��CS2��N3�����е���һ�֣�
��3��ͬһ����Ԫ�أ�Ԫ�صĵ縺������ԭ���������縺��:��<��Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ���һ�������״������ԭ���ǣ���ԭ��3p������ڰ����״̬��������ʧȥ���ӣ�
��4����������Zn2���γ������¶Ե�����Zn2���ɼ���ԭ�¶Ե�������Ե��Ӽ���ų����ñ�Ϊ���Ե��Ӽ���ų⣬�ų��������H��N��H���DZ��
��5�������߳�Ϊapm=a��10-10cm���������=��a��10-10cm��3���þ����а���K+����Ϊ8��[PtCl6]2-���Ӹ���Ϊ8��1/8+6��1/2=4���侧���ܶ�=m/V=4M/[��a��10-10cm��3NA]=4��1030M/(a3 NA)g��cm��3.





