��Ŀ����
����Ŀ������������һ�ֽྻ������������Դ����������(��Ҫ�ɷ�ΪCO��CO2��)��H2��ϣ����ϳɼ״��������������õķ���֮һ��
��1����������Ӧ�Ĵ�������Cu��Zn��Al��Ԫ�ء�д����̬Cuԭ�ӵĺ�������Ų�ʽ��______��
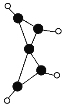
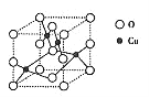
��ͭ��һ�������ᄃ����(�ṹ��ͼ��ʾ)����������Cuԭ����ĿΪ______��
������Oԭ�ӵ���λ��Ϊ______��
��2�����ݵȵ���ԭ����д��CO���ӵĽṹʽ______��
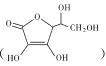
��3���״��������ɵõ���ȩ(HCHO)����ȩ������Cu(OH)2�ļ�����Һ��Ӧ����Cu2O������
�ټ״��е�ȼ�ȩ�ߵö��ԭ����______��
�ڼ״�������ԭ�ӹ�����ӻ�����Ϊsp3��ԭ�Ӹ���Ϊ______��
�ۼ�ȩ���ӵĿռ乹����______��1mol��ȩ��������������ĿΪ______��
�ܼ״���C��H��O����Ԫ�ص縺���ɴ�С��˳��Ϊ______��
��Cu2O���۵��Cu2S�ߵ�ԭ����______��
���𰸡�1s22s22p63s23p63d104s1��[Ar]3d104s1 4 4 ![]() �״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ���� 2 ƽ�������� 3NA O��C��H �����ӵİ뾶С�������ӵ����Ӱ뾶��Cu2O�ľ����ܸ�������Cu2O���۵��Cu2S�ĸ�
�״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ���� 2 ƽ�������� 3NA O��C��H �����ӵİ뾶С�������ӵ����Ӱ뾶��Cu2O�ľ����ܸ�������Cu2O���۵��Cu2S�ĸ�
��������
��1�����ݺ�������Ų����ɼ������ṹ������𣻣�2�����ݵȵ�����ԭ��������𣻣�3����������γɡ��縺�ԡ������ܵ�ԭ��������𣻸��ݼ۲���Ӷ�������ѧ�����ͷ������
��1����Cu��ԭ������Ϊ29�������Ų�ʽΪ1s22s22p63s23p63d104s1��[Ar]3d104s1���ʴ�Ϊ��1s22s22p63s23p63d104s1��[Ar]3d104s1��
�����ݾ���ʾ��ͼ���Կ���Cuԭ�Ӵ��ھ����ڲ�����������Cuԭ����ĿΪ4���ʴ�Ϊ��4��
������ͼʾ֪����ԭ��λ�ڶ��㡢���ϡ����ĺ����ģ�λ�����ĵ�Oԭ����4��Cuԭ����λ���ʴ�Ϊ��4��
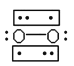
��2�����ݵȵ���ԭ������֪CO��N2Ϊ�ȵ����壬N2���ӵĽṹʽΪ��![]() ����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪ
����Ϊ�ȵ�������ӵĽṹ���ƣ���д��CO�ĽṹʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��3���ټ״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ�������ʼ״��ķе�ߣ��ʴ�Ϊ���״�����֮���γ��˷��Ӽ��������ȩ���Ӽ�ֻ�Ƿ��Ӽ�����������û���γ������
���״������е�Cԭ�Ӻ�Oԭ�Ӽ۲���Ӷ���Ϊ4�����ӻ�����Ϊsp3���ʴ�Ϊ��2��
�ۼ�ȩ������Cԭ��Ϊsp2�ӻ��������µ��Ӷԣ����ӵĿռ乹��Ϊƽ�������Σ�1mol��ȩ�����к���2mol̼��������1mol̼���������ʺ��м������ʵ���Ϊ3mol����ĿΪ3NA�����ʴ�Ϊ��ƽ�������Σ�3NA��
���ǽ�����Խǿ�縺��Խ��ͬ���ڵ�C��O�ǽ�����Oǿ��C����縺��O��C��H���ʴ�Ϊ��O��C��H��
��Cu2O��Cu2Sͬ�������Ӿ��壬����������ͬ�������������ĵ��Ҳ��ͬ���������ӵİ뾶С�������ӵ����Ӱ뾶������Cu2O�ľ����ܴ��۵�ߣ��ʴ�Ϊ�������ӵİ뾶С�������ӵ����Ӱ뾶��Cu2O�ľ����ܸ�������Cu2O���۵��Cu2S�ĸߡ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij̽��С�����ñ�ͪ�������Ӧ(CH3COCH3��Br2![]() CH3COCH2Br��HBr)���о���Ӧ��Ũ���뷴Ӧ���ʵĹ�ϵ����Ӧ���� v(Br2) ͨ���ⶨ�����ɫ��ʧ�����ʱ����ȷ������һ���¶��£��������ʵ�����ݣ�
CH3COCH2Br��HBr)���о���Ӧ��Ũ���뷴Ӧ���ʵĹ�ϵ����Ӧ���� v(Br2) ͨ���ⶨ�����ɫ��ʧ�����ʱ����ȷ������һ���¶��£��������ʵ�����ݣ�
ʵ����� | ��ʼŨ��c/mol��L��1 | ����ɫ��ʧ����ʱ��t/s | ||
CH3COCH3 | HCl | Br2 | ||
�� | 0.80 | 0.20 | 0.0010 | 290 |
�� | 1.60 | 0.20 | 0.0010 | 145 |
�� | 0.80 | 0.40 | 0.0010 | 145 |
�� | 0.80 | 0.20 | 0.0020 | 580 |
����ʵ���������ó��Ľ�������ȷ����(����)
A������c(CH3COCH3)��v(Br2)����
B��ʵ��ں͢۵�v(Br2)���
C������c(HCl)��v(Br2)����
D������c(Br2)��v(Br2)����