��Ŀ����
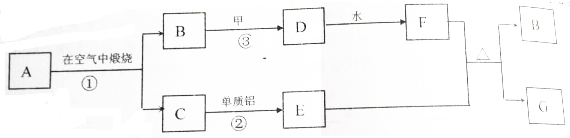
10�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO��SiO2�����ʣ���ҵ�������̿���ȡMnSO4•H2O���������£�
��֪���ٲ��ֽ�����������ȫ����ʱ��pH���±�
| ���������� | Fe3+ | Al3+ | Mn2+ | Mg2+ |
| ��ȫ����ʱ��pHֵ | 3.2 | 5.2 | 10.4 | 12.4 |
��1����������������MnO2ת��ΪMn2+�����ӷ���ʽΪMnO2+SO2=SO42-+Mn2+��
��2����pH��5-6��Ŀ���dz���Fe3+��Al3+����pH��5-6���ӵ��Լ���ѡ��bc���������Լ��������ĸ����
a��NaOH b��MgO c��CaO d����ˮ
��3����2�����ӣ���Ҫ�ǽ�Ca2+��Mg2+ת��Ϊ��Ӧ�����������ȥ��д��MnF2��ȥMg2+�����ӷ�Ӧ����ʽMnF2+Mg2+=Mn2++MgF2���÷�Ӧ��ƽ�ⳣ����ֵΪ7.2��107��
����֪��MnF2��KSP=5.3��10-3�� CaF2��KSP=1.5��10-10��MgF2��KSP=7.4��10-11��
��4��ȡ����MnSO4•H2O����ˮ�������Һ������pH���ָ���Һ�����ԣ�ԭ����Mn2++H2O=Mn��OH��2+2H+�������ӷ���ʽ��ʾ��������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊc��SO42-����c��Mn2+����c��H+����c��OH-����
���� �����̿�֪�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO�����ʣ��������ܽ����SO2+MnO2�TMnSO4������pH������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3��Ȼ���ȥ�����ӣ���ϱ������ݿ�֪CaF2���ܶȻ���С���Ҳ����������ʼ�MnF2���������Ũ�������ȹ��ˣ���ֹ����MnSO4•H2O�ܽ�����٣����Դ������
��1������FeSO4�ڷ�Ӧ�����½�MnO2��ԭΪMnSO4��Fe2+������ΪFe3+��
��2������pH��5��6������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3�����ӹ����в������������ʣ�
��3�����������������д���ӷ���ʽ�û�ѧʽ����Ӧ����ʽΪ��MnF2+Mg2+�TMn2++MgF2��K=$\frac{c��M{n}^{2+}��}{c��M{g}^{2+}��}$��
��4��MnSO4��ǿ�������Σ�ˮ������ԣ�����ʽΪ��Mn2++H2O?Mn��OH��2+2H+������Ũ�ȴ�СΪ����ˮ�����ӣ�ˮ������ӣ����Ե����ӣ����Ե����ӣ�
��� �⣺�����̿�֪�����̿����Ҫ�ɷ�ΪMnO2��������Fe2O3��MgO��Al2O3��CaO�����ʣ��������ܽ����SO2+MnO2�TMnSO4������pH������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3��Ȼ���ȥ�����ӣ���ϱ������ݿ�֪CaF2���ܶȻ���С���Ҳ����������ʼ�MnF2���������Ũ�������ȹ��ˣ���ֹ����MnSO4•H2O�ܽ�����٣���
��1������FeSO4�ڷ�Ӧ�����½�MnO2��ԭΪMnSO4��Fe2+������ΪFe3+���ʡ�������������MnO2ת��ΪMn2+�����ӷ���ʽΪSO2+MnO2�TMn2++SO42-���ʴ�Ϊ��SO2+MnO2�TMn2++SO42-��
��2������pH��5��6������������ij���pH��֪�������ӡ�������ת��Ϊ������������IΪFe��OH��3��Al��OH��3�����ӹ����в������������ʣ����Կɼ������ƺ�����þ������Һ��PH���ʴ�Ϊ��Al��OH��3��Fe��OH��3��a��b��
��3�����������������д���ӷ���ʽ�û�ѧʽ����Ӧ����ʽΪ��MnF2+Mg2+�TMn2++MgF2��K=$\frac{c��M{n}^{2+}��}{c��M{g}^{2+}��}$=$\frac{c��M{n}^{2+}��{c}^{2}��{F}^{-}��}{c��M{g}^{2+}��{c}^{2}��{F}^{-}��}$=7.2��107��
�ʴ�Ϊ��MnF2+Mg2+�TMn2++MgF2��7.2��107��
��4��MnSO4��ǿ�������Σ�ˮ������ԣ�����ʽΪ��Mn2++H2O?Mn��OH��2+2H+������Ũ�ȴ�СΪ����ˮ�����ӣ�ˮ������ӣ����Ե����ӣ����Ե����ӣ���������Ũ�ȴ�СΪ��c��SO42-����c��Mn2+����c��H+����c��OH-����
�ʴ�Ϊ��Mn2++H2O?Mn��OH��2+2H+��c��SO42-����c��Mn2+����c��H+����c��OH-����
���� ���⿼����������ᴿ���ۺ�Ӧ�ã�Ϊ��Ƶ���㣬�������̷������������뷽���������ķ�ӦΪ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�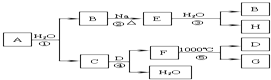 ij��A��Ԫ������Ȼ�������㷺��Ԫ�أ����Ի�����F���ڣ����䵥��A��ʼ������һϵ�л�ѧ��Ӧ��ͼ��ʾ������˵����ȷ���ǣ�������
ij��A��Ԫ������Ȼ�������㷺��Ԫ�أ����Ի�����F���ڣ����䵥��A��ʼ������һϵ�л�ѧ��Ӧ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��Ӧ�١�����H2O���ǻ�ԭ�� | |
| B�� | D��H��Һ��Ӧ��������Һ�����ʳɷ������ֿ��� | |
| C�� | ��ҵ�ϳ���C������Һ��Cl2��Ӧ��ȡƯ�� | |
| D�� | ���F�����ᷴӦ�����Һ����ȡ����A |
| A�� | 0.1mol•L-1 FeSO4��Һ�У�K+��NH4+��MnO4-��ClO- | |
| B�� | ����������Һ�У�Fe3+��Mg2+��SO42-��Br- | |
| C�� | c��H+��=$\sqrt{{K}_{W}}$����Һ�У�K+��Al3+��Cl-��SO42- | |
| D�� | ʹ��̪���ɫ����Һ��Na+��NH2CH2COOH��I-��Ba2+ |
| A�� | H2F+�ĵ���ʽ��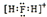 | |
| B�� | ��ԭ�ӵĽṹʾ��ͼ��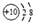 | |
| C�� | ������Ϊ35��������Ϊ45����ԭ�ӣ�${\;}_{35}^{45}$Br | |
| D�� | K2CO3ˮ������ӷ���ʽ��CO32-+2H2O?H2CO3+2OH- |
| A�� | ����ˮ�⣺S2-+2H2O?H2S+2OH- | |
| B�� | ��Ba��OH��2��Һ�еμ�ϡH2SO4��H++OH-�TH2O | |
| C�� | ��CuCl2��Һ�м���H2S��Һ��Cu2++S2-�TCuS�� | |
| D�� | CaCO3����ϡHCl��CaCO3+2H+�TCa2++CO2��+H2O |
| A�� | ҽ�þƾ����õ�����ֲ��;������Ƴɣ�Ũ��ͨ����75% | |
| B�� | ��ˮ�м��뾻ˮ����������ʹ��ˮ���� | |
| C�� | �������ֿɽ��Ʊ걾����������ʹ�����ʱ��Ե����� | |
| D�� | Ѥ���ͷ��̻��������˺��ء��ơ��ơ�ͭ�Ƚ���Ԫ�صĻ����� |
| A�� | ��ϩͨ�����Ը��������Һ������ȡ����Ӧ | |
| B�� | ���Թ��м���2mL5%����ͭ��Һ���ٵμӼ���ϡ����������Һ�����ȣ���������2mL10%��������Һ���ھƾ����ϼ��������ڣ��ɿ�����ɫ���� | |
| C�� | �����������������ͱ���̼������Һ | |
| D�� | ú�ĸ���ú��������ʯ���ѻ��ǻ�ѧ�仯��ʯ�͵ķ����������仯 |