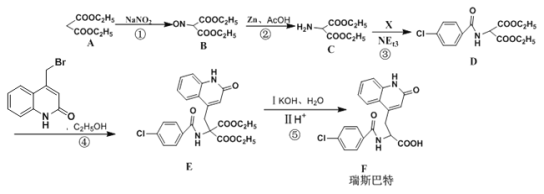
��Ŀ����
����Ŀ���ں��º�ѹ�ܱ�����M����ͼ�ͺ��º����ܱ�����N����ͼ���У��������о�����a molA��a molB����ʼʱ�����������ΪVL���������·�Ӧ���ﵽ��ѧƽ��״̬��2A��?��+ B��?��![]() xC��g�� ��H��0��ƽ��ʱM��A��B��C�����ʵ���֮��Ϊ1��3��4�������жϲ���ȷ����
xC��g�� ��H��0��ƽ��ʱM��A��B��C�����ʵ���֮��Ϊ1��3��4�������жϲ���ȷ����

A. x��2
B. ��N��������ܶ���ͼ����ʾ����A��Bֻ��һ������̬
C. AΪ���壬BΪ�����壬��ƽ��ʱM��N��C�����ʵ������
D. ��A��B��Ϊ���壬ƽ��ʱM��A��ת����С��N��A��ת����
���𰸡�D
��������
����A�����ƽ���B�����ʵ�������nmol����A�����ʵ�������2nmol��C�����ʵ�������xnmol��ƽ��ʱA��B��C�����ʵ����ֱ��ǣ�mol��:a-2n��a-n��xn�����ԣ�a-2n������a-n��=1:3�����n=0.4a����a-2n������xn��=1:4��x=2����ȷ��B��N�Ǻ��º�����������ͼIII��֪��������ܶ�������˵����������������ӣ���A��B�������壬����������ܶȻ�һֱ���䣬����A��B��ֻ��һ������̬����ȷ��C��AΪ���壬BΪ�����壬��÷�Ӧ�Ƿ�Ӧǰ���������ʵ�������Ŀ��淴Ӧ�����º�ѹ����º��ݴﵽ��ƽ���ǵ�Ч�ģ�����ƽ��ʱM��N��C�����ʵ�����ȣ���ȷ��D����A��B��Ϊ���壬Mƽ����������ѹǿ����N������������M�����õ�N������ƽ��״̬����Ҫ��Сѹǿ��ƽ�������ƶ���M��A��ת���ʼ�С����N��A��ת������ͬ������ԭƽ��ʱM��A��ת���ʴ���N��A��ת���ʣ�����ѡD��

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д�