��Ŀ����
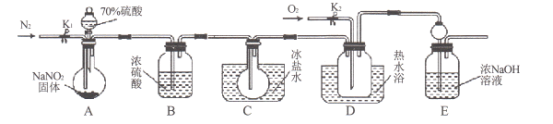
����Ŀ����ҵ����Ҫ�����ж�±ˮ���д��幤��������ij��ѧС���ͬѧΪ�˽�ӹ�ҵ�����ᴿ��ķ������������ʵ��װ�ã�

�й����ϣ�Br2�е�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ�
(1)ͼ��ʢװ��ҵ�������������______���ձ�A����ˮԡ������Ϊ______��
(2)��ͼ���Ӻ�װ�ã����װ�������Բ�װ���Լ���Ȼ��________���ٿ�ʼ��A���ȡ�C��Һ��������ɫΪ______��
(3)ʵ���ҷ����廹�������ܼ���ȡ�������п������������ȡ������ _________��
A.�Ҵ� B.���Ȼ�̼ C.������Һ D.�ѻ�����
(4)Br2������Na2CO3��Һ���յ���������ΪNaBrO3����D�з�����Ӧ�Ļ�ѧ����ʽΪ__________��
���𰸡�Բ����ƿ ʹҺ�����Ⱦ��ȣ����ڿ����¶� ���������а�ͼ�м�ͷ����ͨ����ˮ �����ɫ�����ɫ B 3Br2+6Na2CO3+3H2O��NaBrO3+5NaBr+6NaHCO3
��������
ͨ����ˮԡ����ҵ����ȣ����Ϊ������Ȼ��ͨ���������뵽װ�б���˫����ƿ�У������ӷ���Ϊ����������������Ի��������Ⱦ������±�ص���������������ʷ�Ӧ�����ʣ��ù���Na2CO3��Һ���գ������绯��Ӧ����NaBrO3��NaBr��Na2CO3���ΪNaHCO3��
(1)ͼ��ʢװ��ҵ���������Բ����ƿ��ˮԡ���ȵ��ŵ���ʹҺ�����Ⱦ��ȣ����ڿ����¶ȣ�
(2)��ͼ���Ӻ�װ�ã����װ�������Բ�װ���Լ���Ȼ���������ͨ����ȴˮ������ʱ��Ϊ�������������ˮӦ���¿ڽ����Ͽڳ�����ͨ����ˮ�ķ���Ϊͼ�м�ͷ����Ȼ���A���м��ȣ�ʹҺ�������������¶�59�棬ʹ�嵥�ʻӷ���ͨ�������õ��������ɫ�����ɫ��Һ̬�嵥�ʣ�
(3)A.���ھƾ��е��ܽ�ȴ�����ˮ�е��ܽ�ȣ����ƾ���ˮ���ܣ���������ȡ����A���������⣻
B.����CCl4�е��ܽ�ȴ�����ˮ�е��ܽ�ȣ�������CCl4����Ӧ��ˮ��CCl4�������ܣ�CCl4��������ȡ����B�������⣻
C.�嵥�����봿����Һ��Ӧ������Һ�壬��������ȡ����C���������⣻
D.�ѻ������к���ϩ��������Һ�巢���ӳɷ�Ӧ�������壬��������ȡ����D���������⣻
�ʺ���ѡ����B��
(4)Br2�����Na2CO3��Ӧ����NaBrO3��NaBr��NaHCO3�����ݵ����غ㡢ԭ���غ㣬�ɵø÷�Ӧ�Ļ�ѧ����ʽΪ3Br2+6Na2CO3+3H2O��NaBrO3+5NaBr+6NaHCO3��