��Ŀ����
����Ŀ����֪�����ڱ���ǰ�����ڵ�����Ԫ��A��B��C��D��E��F�˵����������������Aԭ�Ӻ���������δ�ɶԵ��ӣ�������B2E�ľ���Ϊ���Ӿ��壬Eԭ�Ӻ����M���������ԳɶԵ��ӣ�CԪ���ǵ��к�����ߵĽ���Ԫ�أ�D���ʵ��۵���ͬ����Ԫ���γɵĵ���������ߵģ�F2�����Ӻ��������Ӿ������������������Ϣ���ش��������⣺
��1��A��B��C��D�ĵ�һ��������С�����˳��Ϊ______����Ԫ�ط��ű�ʾ��
��2��B���Ȼ�����۵��D���Ȼ�����۵�ߣ�������______��
��3��E�������������ӵĿռ乹����______����______���ӣ�����ԡ����Ǽ��ԡ���
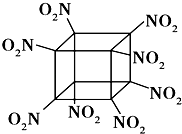
��4��Fԭ�ӵļ۲�����Ų�ʽ��_____��
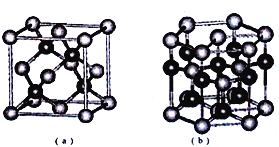
��5��E��F�γ�ij�ֻ���������ͼ��ʾ���־���ṹ����ɫ���ʾFԭ�ӣ����仯ѧʽΪ_____����a����Eԭ�ӵ���λ��Ϊ______�����ڣ�b���Ľṹ��ȡ��һ��ƽ����������Ϊ��������ƽ��һ�������к���_____��Fԭ�ӡ��ṹ��a���루b���о�����ԭ�ӿռ���������ȣ���a��________��b�����>����<����=����
���𰸡� ![]() NaCl Ϊ���Ӿ����SiCl4Ϊ���Ӿ��� ƽ���������� �Ǽ���
NaCl Ϊ���Ӿ����SiCl4Ϊ���Ӿ��� ƽ���������� �Ǽ��� ![]() ZnS 4 2 >
ZnS 4 2 >
��������C�ǵؿ��к�����ߵĽ���Ԫ��,��C��AlԪ����Aԭ�Ӻ�����3��δ�ɶԵ��ӣ���A��ԭ������С��C(Al)����Aԭ�Ӻ�������Ų�ʽΪ1s22s22p3��A�ǵ�(N)Ԫ����Eԭ�Ӻ����M���������ԳɶԵ�����Eԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p4��E��SԪ����������B2E�ľ���Ϊ���Ӿ�����B��+1��Ϊ��IA��Ԫ����B��ԭ���������ڵ�Ԫ�ء�С��AlԪ��������B��NaԪ�������ԭ��������֪D���ڵ���������D���ʵľ����۵���ͬ�����γɵĵ���������ߵ�������D��SiԪ����F2+���Ӻ�����Dz���Ӿ��ѳ�����Fԭ����������SԪ�������ڵ���������F2+���Ӻ�������Ų�ʽΪ1s22s22p63s23p63d10����Fԭ�Ӻ��������Ϊ30����F��ZnԪ����
��1��A��B��C��D�ֱ���N��Na��Al��SiԪ�أ�ͬһ�����У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�ͬһ�����У�Ԫ�صĵ�һ����������ԭ���������������С�����Ե�һ�����ܣ�Na��Al��Si��P��P��N�����Ե�һ�����ܴ�С˳��ΪNa��Al��Si��N��
�ʴ�Ϊ��Na��Al��Si��N��
��2��B���Ȼ�����NaCl��D���Ȼ�����SiCl4��NaCl�����Ӿ��壬SiCl4�Ƿ��Ӿ��壬���Ӿ�����۵���ڷ��Ӿ�����
�ʴ�Ϊ��NaCl�����Ӿ���,SiCl4�Ƿ��Ӿ��壻
��3��E��SԪ����SO3����Sԭ�Ӻ���3�������Ҳ����µ��Ӷԣ�����Ϊƽ���������νṹ��SO3������������������غϣ�Ϊ�Ǽ��Է�����
�ʴ�Ϊ��ƽ���������Σ��Ǽ��ԣ�
��4��FΪZnԪ�أ�ZnΪ30��Ԫ�أ�ԭ�Ӻ�������Ų�ʽΪ[Ar]3d104s2���۲�����Ų�ʽΪ��3d104s2���ʴ�Ϊ��3d104s2��
��5��E��SԪ��,F��ZnԪ����Sԭ�Ӹ���=8��![]() +6��
+6��![]() =4��Znԭ�Ӹ���=4�����Ըþ�����S��Znԭ�Ӹ���֮��=4:4=1:1�������仯ѧʽΪZnS����aͼ�У��Զ���Sԭ��Ϊ������S���������Znԭ����4�����ֲ���Sԭ����Χ�����ڵ��������ڣ���S��λ��Ϊ4����b�Ľṹ��ȡ��һ��ƽ����������Ϊ������������ͼ��ʾ�Ľṹ
=4��Znԭ�Ӹ���=4�����Ըþ�����S��Znԭ�Ӹ���֮��=4:4=1:1�������仯ѧʽΪZnS����aͼ�У��Զ���Sԭ��Ϊ������S���������Znԭ����4�����ֲ���Sԭ����Χ�����ڵ��������ڣ���S��λ��Ϊ4����b�Ľṹ��ȡ��һ��ƽ����������Ϊ������������ͼ��ʾ�Ľṹ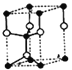 ���������ʾZnԭ�ӣ������к���Znԭ����Ϊ8��
���������ʾZnԭ�ӣ������к���Znԭ����Ϊ8��![]() +1=2����bͼ�������ӵĶѻ���ʽΪ�������ܶѻ���ͼa��������֮���γ������������ѻ���ԭ�ӿռ���������bͼ�������ӵĶѻ���ʽ��ͬ������aͼ�о����к���4��п���ӣ���bͼ������2��п���ӣ��ʽṹa�е�ԭ�������ʴ��ڽṹb�е�ԭ�ӿռ������ʡ�
+1=2����bͼ�������ӵĶѻ���ʽΪ�������ܶѻ���ͼa��������֮���γ������������ѻ���ԭ�ӿռ���������bͼ�������ӵĶѻ���ʽ��ͬ������aͼ�о����к���4��п���ӣ���bͼ������2��п���ӣ��ʽṹa�е�ԭ�������ʴ��ڽṹb�е�ԭ�ӿռ������ʡ�
�ʴ�Ϊ��ZnS��4��2������

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�����Ŀ����ѧ��Ӧԭ���ڻ���������ʵ�������Ź㷺����Ҫ��Ӧ�á�
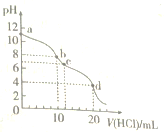
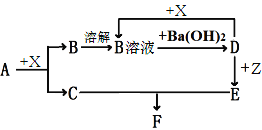
��.���ú��̷�ˮ(��Ҫ��Mn2����SO![]() ��H����Fe2����Al3����Cu2��)���Ʊ������ܴ��Բ���̼����(MnCO3)������һ�ֹ����������£�
��H����Fe2����Al3����Cu2��)���Ʊ������ܴ��Բ���̼����(MnCO3)������һ�ֹ����������£�

��֪ijЩ������ȫ������pH���±���
������ | Fe(OH)3 | Al(OH)3 | Cu(OH)2 | Mn(OH)2 | CuS | MnS | MnCO3 |
������ȫʱ��pH | 3.2 | 5.4 | 6.4 | 9.8 | ��0 | ��7 | ��7 |
�ش��������⣺
��1�����̢��У���������W����Ҫ�ɷ���______________________��
��2�����̢��У�������Ӧ�����ӷ���ʽ��______________________��
��3�����̢��У������ɵ�����J��ʹ����ʯ��ˮ����ǣ�������MnCO3�ķ�Ӧ�����ӷ���ʽ��_______________________��
��4����MnCO3���Ƶ���Ҫ�Ĵ���MnO2��2MnCO3��O2===2MnO2��2CO2�����ڿ����м���460.0 g MnCO3���õ�332.0 g��Ʒ������Ʒ������ֻ��MnO����ò�Ʒ��MnO2������������________(�ðٷ�����ʾ��С�������1λС��)��
��.�����£�Ũ�Ⱦ�Ϊ0.1 mol��L��1������������Һ��pH���±���
���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN/span> | C6H5ONa |
pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 | 11.3 |
��1����������Һ�е������ӣ����H��������ǿ����_______________________��
��2�����ݱ��������жϣ�Ũ�Ⱦ�Ϊ0.01 mol��L��1���������ʵ���Һ�У�������ǿ����________(�����)��
A. HCN�� B. HClO�� C. C6H5OH�� D. CH3COOH E. H2CO3
��.��֪:CO(g)+H2O(g)![]() CO2(g)+H2(g)����H = Q kJ��mol-1��ƽ�ⳣ�����¶ȱ仯���±���ʾ:
CO2(g)+H2(g)����H = Q kJ��mol-1��ƽ�ⳣ�����¶ȱ仯���±���ʾ:
�¶�/�� | 400 | 500 | 850 |
ƽ�ⳣ�� | 9.94 | 9 | 1 |
��ش���������:
��1��������Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ___,�÷�Ӧ��Q____(����>������<��)0��
��2��850 ��ʱ,�����Ϊ10 L�ķ�Ӧ����ͨ��һ������CO��H2O(g),����������Ӧ,CO��H2O(g)��Ũ�ȱ仯��ͼ��ʾ,��0~4 minʱƽ����Ӧ����v(CO)=____��
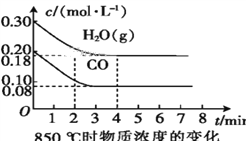
��3������500 ��ʱ����������Ӧ,��CO��H2O(g)����ʼŨ�Ⱦ�Ϊ0.020 mol��L-1,��������,CO�����ת����Ϊ____��
��4������850 ��ʱ����������Ӧ,����ʼʱCO��H2O(g)��Ϊ1 mol,����ˮ�������������Ϊx,ƽ��ʱCO��ת����Ϊy,���Ƶ�y��x�仯�Ĺ�ϵʽ:____��
����Ŀ�����и��������ܴ������棬�ҵ������Լ���Ӧ�����ӷ���ʽ��д��ȷ����(�� ��)
ѡ�� | ���� | �����Լ� | ������Ӧ�����ӷ���ʽ |
A | Fe3����I����Cl�� | NaOH��Һ | Fe3����3OH��===Fe(OH)3�� |
B | K����NH3��H2O��CO | ͨ������CO2 | 2OH����CO2===CO |
C | H����Fe2����SO | Ba(NO3)2��Һ | SO |
D | Na����Al3����Cl�� | ��������ʯ��ˮ | Al3����3OH��===Al(OH)3�� |
A. A B. B C. C D. D