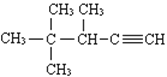
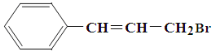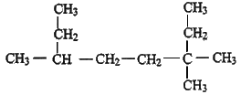��Ŀ����
����Ŀ����������±�����Ĵ�����ͨ�����������̽��������±���������ʣ����Դﵽ��һ������������ͨ��Ч����
I����֪��NaBr+H2SO4(Ũ) ![]() NaHSO4+ HBr CH3CH2OH+HBr
NaHSO4+ HBr CH3CH2OH+HBr ![]() CH3CH2Br+H2O
CH3CH2Br+H2O
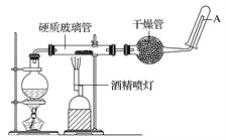
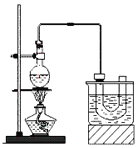
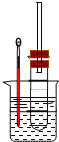
������ķе�38.4����ʵ�����Ʊ������飨CH3CH2Br����װ�úͲ������£�
�����װ�õ������ԣ���װ��ͼ��ʾ��U�ιܺʹ��ձ��м����ˮ��
����Բ����ƿ�м���10mL95%�Ҵ���28mL78%Ũ���ᣬȻ�������ϸ��13g�廯�ƺͼ������Ƭ��
��С�ļ��ȣ�ʹ���ַ�Ӧ��
�ش��������⣺
��1��Ϊ�˸��õĿ��Ʒ�Ӧ�¶ȣ�����ͼʾ��С����ȣ����õļ��ȷ�ʽ��_______________��
��2��Ũ�������ǿ�����ԣ���������ԭ������HBrΪBr2������U�ι��д��Ƶ���������ػ�ɫ��Ϊ�˳�ȥ�ֲ�Ʒ�е�����Br2����ѡ�������Լ��еģ�______������ţ�
A��NaOH��Һ B��H2O
C��Na2SO3��Һ D��CCl4
����ʱ�������Ҫ����������______________�����������ƣ���Ҫ��һ���Ƶô����������飬����ˮϴ��Ȼ�������ˮCaCl2���ٽ���_________����������ƣ���
II���������ڲ�ͬ�ܼ�����NaOH�ɷ�����ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ���ͼʵ��װ��(����̨���ƾ�����)��֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
��1�����Թ��м���5 mL 1 mol/L NaOHˮ��Һ��5 mL �����飬���Թ���ͼ�̶����ȡ�
���Թܿڰ�װһ�����ܵ�������_____________________________��
���������������Ҵ��Ľṹ�����õIJ�����___________________��___________________��
��2�����Թ��м���5 mL NaOH�Ҵ���Һ��5 mL �����飬���Թ���ͼ�̶����ȡ�
����д���÷�Ӧ�Ļ�ѧ����ʽ____________________________________________________��
��Ϊ֤����������NaOH�Ҵ���Һ�з���������ȥ��Ӧ������Ƶ�ʵ�鷽���У���Ҫ�������___________�������װ����ͼ��ʾ��������ͨ�����Ը��������Һǰ��һ��ʢˮ���Թܣ���������_____________��
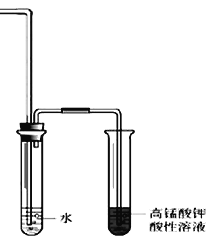
���𰸡�ˮԡ���� C ��Һ©�� ���� �������� ������� �˴Ź������� CH3CH2Br+NaOH![]() CH2=CH2����NaBr��H2O ��ϩ ��ȥ��ϩ�л��е��Ҵ�����
CH2=CH2����NaBr��H2O ��ϩ ��ȥ��ϩ�л��е��Ҵ�����
��������
I.��1��Ϊ�˸��õĿ����¶ȣ������ˮԡ���ȵķ�����
��2��U�ܵõ��������飬�������л����壬����ʱע���������������ʽǶȿ��ǣ���Һ�õ���Һ©���������鲻����ˮ��ˮϴ���Һ���룬�ټ�����ˮCaCl2�����������������
II.��1�����������ӷ����ó����ܽ������������������������ʧ��
�ڸ����Ҵ����ӽṹ����������ԭ��ѡ���ⷽ����
��2����������Ϊ±������ͬNaOH�Ҵ���Һ�ڼ��������»ᷢ����ȥ��Ӧ���䷴Ӧ����ʽΪ��CH3CH2Br+NaOH![]() CH2=CH2����NaBr��H2O��
CH2=CH2����NaBr��H2O��
�����۷���ȡ����Ӧ������ȥ��Ӧ����Һ�ж������Br-�������ɵ��л��ﲻͬ����Ӧ�������ɵ��л���Ҵ�������ˮ������ϩ������ˮ��
I.��1��Ϊ�˸��õĿ����¶ȣ�����ͼʾ��С������⣬�����ˮԡ���ȵķ�����
��2����U������Br2��A.�������ܹ����������Ʒ�Ӧ����A����B.��������嵥�ʾ�������ˮ�������з��룬��B����C.Na2SO3���巢��������ԭ��Ӧ��Na2SO3�ɳ�ȥ�壬��C��ȷ��D.��������鶼���������Ȼ�̼�����ܽ����߷��룬��D����������������Һϴ�Ӻ���Ȼ���Һ����ȡ��Һʹ�õ���Ҫ�����Ƿ�Һ©����Ҫ��һ���Ƶô�����C2H5Br������ˮϴ����Һ���ټ�����ˮCaCl2�����÷е㲻ͬ�ٽ���������룻
II.��1����������ķе�38.4�����������ӷ����ó����ܽ������������������������ʧ��
���Ҵ����ӽṹ����������ԭ�ӣ����ǵı�Ϊ3:2:1�����ú˴Ź������ɼ�⣬Ҳ���ú������⣻
��2����������Ϊ±������ͬNaOH�Ҵ���Һ�ڼ��������»ᷢ����ȥ��Ӧ���䷴Ӧ����ʽΪ��CH3CH2Br+NaOH![]() CH2=CH2����NaBr��H2O��
CH2=CH2����NaBr��H2O��
�����۷���ȡ����Ӧ������ȥ��Ӧ����Һ�ж������Br-�������ɵ��л��ﲻͬ�������鷢����ȥ��Ӧ������ϩ������Ӧ�������ɵ��л�����ϩ��������ϩ�ɸ�������ʹ���Ը��������ɫ��ԭ�����У���ͬʱ�Ҵ�Ҳ��ʹ���Ը��������ɫ������Ҫ��ȥ��ϩ�е��Ҵ����Ҵ�������ˮ������ϩ��������ˮ����������ͨ�����Ը��������Һǰ��һ��ʢˮ���Թܣ��������dz�ȥ��ϩ�л��е��Ҵ�������