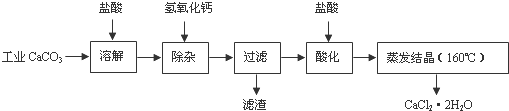
��Ŀ����
14������һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��Al3+��Fe3+��Mg2+��Ba2+��NH4+��Cl-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺��1����һ�ݼ���AgNO3��Һ�г���������
��2���ڶ��ݼ�����NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ�ͬʱ�õ���Һ�ף�
��3���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��
��4�������ݼ�����BaCl2��Һ�ð�ɫ��������������������ϴ�ӡ����������Ϊ11.65g��{��֪��NaAlO2+2H2O�TNaHCO2+Al��OH��3��}����������ʵ��ش�
��1��һ�������ڵ�������Fe3+��Mg2+��CO32-��Ba2+������ȷ���Ƿ���ڵ�������Cl-��
��2����ȷ����Һ�п϶����ڵ����Ӽ���Ũ�ȣ��ɲ���������
���ӷ���NH4+��Ũ��0.2mol/L��
���ӷ���Al3+��Ũ��0.2mol/L��
���ӷ���SO42-��Ũ��0.5mol/L��
��3����ȷ��K+�Ƿ�����ǣ���ǡ������жϵ������Ǹ��ݵ���غ㣬��Һ��һ�����ڼ����ӣ�
���� ��1����һ�ݼ��뼸��AgNO3��Һ���г�����������Һ�п��ܺ���Cl-��CO32-��SO42-��
��2���ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ��������ǰ�����һ����笠����ӣ�笠����ӵ����ʵ�����0.02mol���������ɣ�һ������Fe3+��Mg2+��
��3���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��˵��ԭ��Һ��һ������Al3+��������ӹ����֪һ��������CO32-��1.02gΪ�������������������ʵ���Ϊ��$\frac{1.02g}{102g/mol}$=0.01mol��˵��ÿ����Һ�к���0.02molAl3+��
��4�������ݼ�����BaCl2��Һ�ð�ɫ�������ð�ɫ����Ϊ���ᱵ��˵��ԭ��Һ��һ������SO42-����һ�������ڱ����ӣ���������������ϴ�ӡ����������Ϊ11.65g�������ᱵ�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol��˵��ÿ����Һ�к���0.05molSO42-��
0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+���ݴ˶Ը�ѡ������жϣ�
��� �⣺��һ�ݼ��뼸��AgNO3��Һ���г�����������Һ�п��ܺ���Cl-��CO32-��SO42-��
�ڶ��ݼӹ���NaOH��Һ���Ⱥ�ֻ�ռ�������0.02mol���������ɣ��������ǰ�����һ����笠����ӣ�笠����ӵ����ʵ�����0.02mol���������ɣ�һ������Fe3+��Mg2+���ڼ���Һ��ͨ�����CO2�����ɰ�ɫ���������������ˡ�ϴ�ӡ����գ�����Ϊ1.02g��˵��ԭ��Һ��һ������Al3+��������ӹ����֪һ��������CO32-��1.02gΪ�������������������ʵ���Ϊ��$\frac{1.02g}{102g/mol}$=0.01mol��˵��ÿ����Һ�к���0.02molAl3+��
�����ݼ�����BaCl2��Һ�ð�ɫ�������ð�ɫ����Ϊ���ᱵ��˵��ԭ��Һ��һ������SO42-����һ�������ڱ����ӣ���������������ϴ�ӡ����������Ϊ11.65g�������ᱵ�����ʵ���Ϊ��$\frac{11.65g}{233g/mol}$=0.05mol��˵��ÿ����Һ�к���0.05molSO42-��
0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+��
��1�����ݷ�����֪��һ�����ڵ�����Ϊ��K+��NH4+��Al3+��SO42-��һ�������ڵ�����Ϊ��Fe3+��Mg2+��CO32-��Ba2+�����ݷ�����֪����ȷ���Ƿ����Cl-��������Һ�п��ܴ���Cl-��
�ʴ�Ϊ��Fe3+��Mg2+��CO32-��Ba2+��Cl-��
��2��笠����ӵ����ʵ�����0.02mol������0.02molAl3+������0.05molSO42-��0.02molAl3+���������Ϊ0.06mol��0.02mol笠����Ӵ���0.02mol����ɣ���0.05molSO42-���������Ϊ0.1mol�����ݵ���غ��֪��˵����Һ��һ��������������K+����Һ���Ϊ0.1L��������Ũ�ȷֱ�Ϊ0.2mol/L��0.2mol/L��0.5mol/L��
�ʴ�Ϊ��NH4+��0.2mol/L��Al3+��0.2mol/L��SO42-��0.5mol/L��
��3����Һ������ɵ������ʵ���Ϊ��n��NH4+��+3n��Al3+��=0.02mol+3��0.02mol=0.08mol��
����ɵ������ʵ���Ϊ��2n��SO42-��=0.1mol�����ݵ���غ㣬��Һ��һ�����ڼ����ӣ����ڿ��ܴ��������ӣ�������ӵ����ʵ�������Ϊ��0.1mol-0.08mol=0.02mol��c��K+����0.2mol��l-1���ʴ�Ϊ���ǣ����ݵ���غ㣬��Һ��һ�����ڼ����ӣ�
���� ���⿼�鳣�����ӵļ��飬��Ŀ�Ѷ��еȣ����ö���ʵ��Ͷ�������������ϵ�ģʽ�������˽����Ѷȣ�ͬʱ�漰���ӹ��桢���ӷ�Ӧ�ȶ��ǽ�����ע�����Ϣ��������K+��ȷ���׳���ʧ��

 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�| A�� | ��״���£�1.12LSO3������ԭ������0.2NA | |
| B�� | 3.9gNa2O2����������CO2ʱת�Ƶ�������0.05NA | |
| C�� | 20g��ˮ�к���������Ϊ8NA | |
| D�� | 28g��ϩ�ͻ����飨C4H8���Ļ�������к��е�̼ԭ����Ϊ3NA |
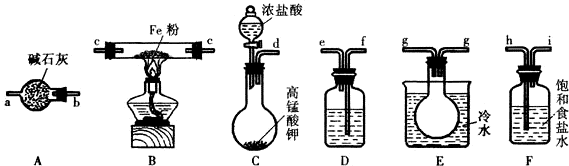
 ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ��
ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ����1��ϡ����Ӧ����1�У���д������ţ���
��2����ʵ��ͨ������A��B��C�������أ��������еĿ����ž����ٹرտ���B������AC�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п��������ɷ�ֹ���ɵ�������������������
��3����ʵ��ʹ�����ۣ�����Ӧ���ʿ���̫���⣬�����ܻ���ɵIJ�����������۽��뵼�ܴӶ��������ܣ��˿�ɾȥ
��4����FeSO4��Һ�м��루NH4��2SO4������Ʊ���������茶���[��NH4��2SO4•FeSO4•6H2O]��ʽ��Ϊ392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ�ӣ�NH4��2SO4•FeSO4•6H2O�ֲ�Ʒ�����з���������ʵ���D��
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol•L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ��յ�����������һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ��
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ$\frac{980c}{a}$��100%������ĸac�������ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������BC��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�
| A�� | ̼��ĵ���Ϊ���ȹ��̣�����̼���������ᷴӦ���� | |
| B�� | ��֪NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-57.3kJ•mol-1���� 40.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�����������57.3 kJ | |
| C�� | ͨ��NaOH��aq��+HCl��aq��=NaCl��aq��+H2O��l����H=-57.3kJ•mol-1����֪ 1molH2O��l����ȫ������Ҫ����57.3kJ | |
| D�� | ���ȷ�ӦΪ���ȷ�Ӧ |