��Ŀ����
19��ҽ���Ȼ��ƿ������������ơ�������������ҩ��Թ�ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ�����ҽҩ����ˮ���Ȼ��ƣ�CaCl2•2H2O����������Ϊ97.0%-103.0%������Ҫ������ͼ��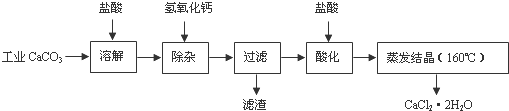
��1�����Ӳ����Ǽ����������ƣ�������Һ��pHΪ8.0-8.5���Գ�ȥ��Һ�е�����Al3+��Fe3+������Fe��OH��3�Ƿ������ȫ�����������ȡ�����ϲ���Һ���Թ��У��μ�KSCN��Һ����������Ѫ��ɫ�������Fe��OH��3������ȫ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٽ���Һ�е�����Ca��OH��2ת��ΪCaCl2���ڷ�ֹCa2+������ʱˮ�⣻��ֹ��Һ���տ�����CO2��
��3���ⶨ��Ʒ��Cl- �����ķ����ǣ�a����ȡ0.7500g��Ʒ���ܽ⣬��250mL����ƿ�ж��ݣ�b����ȡ25.00mL������Һ����ƿ�У�c����0.05000mol•L-1AgNO3��Һ�ζ����յ㣬����AgNO3��Һ�����ƽ��ֵΪ20.39mL��
�ټ���������Ʒ��CaCl2•2H2O����������Ϊ99.9%��
�����������취�ⶨ����Ʒ��CaCl2•2H2O������������ʱ����100.0%���ⶨ�����в��������ɺ��ԣ��������ԭ������Ʒ�д���������NaCl��������CaCl2.2H2Oʧˮ��
���� ��ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ��������������ȫ�ܽ������Ȼ��ơ��Ȼ����Լ��Ȼ��ơ��Ȼ����ȣ���������������Һ���Գ�ȥ��Һ��������Fe3+��Ȼ�������������������ᾧ�ɵõ�CaCl2•2H2O��
��1��Fe3+��KSCN��Ӧ���ɺ�ɫ����Fe��SCN��3������Fe3+�Ƿ���ڵģ�ѡ��KSCN��Һ��
��2�����ڳ��ӹ����м�����Ca��OH��2���ʼ�����������Һ�е�����Ca��OH��2��Ӧʹ��ת��ΪCaCl2����Ca��OH��2�����տ����е�CO2������CaCO3�������ʼ������ỹ���Է�ֹ��Һ���տ�����CO2��Ca��OH��2���տ����е�CO2������CaCO3������������մ�����CO2 �ᵼ��������������ƫ�ͣ�
��3���ٸ��ݵ���ζ��յ������Ȼ��������ʵ����������ĵ������������ʵ�����һ��ϵ������������������ʵ�������n��AgCl��=2n��CaCl2.2H2O����
�ݴ˿������ʵ���ϵ�CaCl2.2H2O�����ʵ������������������ע����������Ǵ�250mol��ȡ25ml�������ڼ���ʱҪע����һ�㣻
����Ʒ�д���������NaCl������ n��AgCl��2n��CaCl2.2H2O����֪��CaCl2.2H2O�����ʵ�������ͬ����CaCl2.2H2Oʧˮ���·�ĸ��С��ֵƫ��
��� �⣺��ҵ̼��ƣ���������Na+��Al3+��Fe3+�����ʣ��������������ȫ�ܽ������Ȼ��ơ��Ȼ����Լ��Ȼ��ơ��Ȼ����ȣ���������������Һ���Գ�ȥ��Һ��������Fe3+��Ȼ�������������������ᾧ�ɵõ�CaCl2•2H2O��
��1��Fe3+��KSCN��Ӧ���ɺ�ɫ����Fe��SCN��3������Fe3+�Ƿ���ڵģ�ѡ��KSCN��Һ��
�ʴ�Ϊ��ȡ�����ϲ���Һ���μ�KSCN��Һ����������Ѫ��ɫ�������Fe��OH��3 ������ȫ��
��2���ữ�����Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٽ���Һ�е�����Ca��OH��2ת��ΪCaCl2�� �ڷ�ֹCa2+������ʱˮ�⣻�۷�ֹ��Һ���տ�����CO2��
�ʴ�Ϊ������Һ�е�����Ca��OH��2ת��ΪCaCl2����ֹCa2+������ʱˮ�⣻��ֹ��Һ���տ�����CO2��
��3������Ʒ��n��Cl-��=0.05000mol•L-1��0.02039L��10=0.010195mol������n��AgCl��=2n��CaCl2.2H2O������n��CaCl2.2H2O��=0.0050975mol������m��CaCl2.2H2O��=0.0050975mol��147g/mol=0.7493325g�����У�$\frac{0.7493225g}{0.7500g}$��100%=99.9%��
�ʴ�Ϊ��99.9%��
����Ʒ�д���������NaCl�ᵼ��CaCl2.2H2O�����ʵ�������ͬ����CaCl2.2H2Oʧˮ���·�ĸ��С��ֵƫ��
�ʴ�Ϊ����Ʒ�д���������NaCl��������CaCl2.2H2Oʧˮ��
���� ���⿼�������к����IJⶨ���漰ʵ��Ļ���������ʵ��������ѡ��ʵ�����������������뼰�����ⶨ�ļ���ȣ�ע�����ӵļ��鷽���ͳ���������ʹ�ã���Ʒ���ȵķ���Ҫע����Һ�п��ܷ����ķ�Ӧ��ע����Ч�������⣬��Ŀ�ۺ��Խ�ǿ���Ѷ��еȣ�

 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д���������İٷֺ���һ������
�ڷ�Ӧ��ת����һ������
��������IJ���һ������
�ܷ�Ӧ��Ũ��һ������
������Ӧ����һ�������淴Ӧ����
��ʹ���˺��ʵĴ�����
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �ܢ� |
| A�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1012��ˮ��Һ�У�NH4+��Al3+��NO3-��Cl- | |
| B�� | c��Fe3+��=0.1 mol/L����Һ�У�K+��ClO-��SO42-��SCN- | |
| C�� | pH=1����Һ�У�Fe2+��NO3-��SO42-��Na+ | |
| D�� | ˮ�������c��H+��=10-12 mol/L����Һ�У�Ca2+��K+��Cl-��HCO3- |
| A�� | NaOH | B�� | Ba��OH��2 | C�� | Fe��OH��3 | D�� | NH3•H2O |
 �������ǻ����飬�����Ķ��ȴ�����4�֣�
�������ǻ����飬�����Ķ��ȴ�����4�֣�