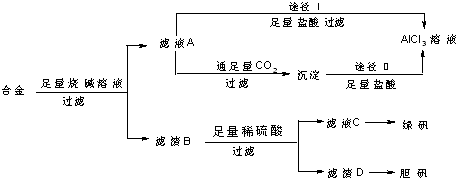
��Ŀ����
2���ᡢ�����Һ�ܹ����磬����Ϊ��������ˮʱ������Ϊ�����ƶ������������ӣ��������Һ����������������ɵ��������������ʱ���ɵ�H+����������1���������ʵĵ��뷽��ʽ���£�HClO4�TH++ClO4-�� Ba��OH��2�TBa2++2OH-��
Fe2��SO4��3�T2Fe3++3SO42-�� KHSO4�TK++H++SO42-��
�����������HClO4��д��ѧʽ����
��2�������������õ�����������������ᡱָ���ᡢ��������ᣬ�����ָ�ռ�ʹ��
�ٴ����ʵķ���Ƕȿ�����ǡ����һ�������Ǵ��
�����������мȲ��ǵ����Ҳ���Ƿǵ���ʵ������ᡢ���ᣮ
��3��д������������ˮ��Һ��ĵ��뷽��ʽ����NaHCO3NaHCO3�TNa++HCO3-��H2SO4H2SO4�T2H++SO42-
��4������˵�ɽ���������KHSO4��NaHCO3��Ϊͬһ�������Ϊ����������Ҫ��KHSO4�� NaHCO3���ɽ��������Ӻ���ʽ�����ӹ��ɣ���������ʽ�Σ�����˵�ɽ���������KHSO4��H2SO4��Ϊͬһ�������Ϊ����������Ҫ��KHSO4��H2SO4������ˮ��Һ�е����H+��ˮ��Һ�����ԣ�
��5������NaHSO4��Ba��OH��2����Һ�а����ʵ���֮��Ϊ2��1��ϣ��÷�Ӧ�����ӷ���ʽΪ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O��
����NaHSO4��Ba��OH��2����Һ�а����ʵ���֮��Ϊ1��1��ϣ��÷�Ӧ�����ӷ���ʽΪ��H++SO42-+Ba2++OH-�TBaSO4��+H2O��
���� ��1�����������������ȫ���������ӵĻ�����Ϊ�ᣬ�������������ȫ�������������ӵĻ��������ڼ����ָ�ɽ������Ӻ����������ɵĻ����
��2���ٴ���ΪNa2CO3�����ɽ������Ӻ�������ӹ��ɵģ�
�ڻ����Ȳ��ǵ���ʣ��ֲ��Ƿǵ���ʣ�
��3��̼�����ƺ���������Һ����ȫ���룻
��4�������ӹ�����ȷ��KHSO4�� NaHCO3����𣻸���KHSO4��H2SO4��ˮ��Һ�е�����������Լ���Һ���������жϣ�
��5�����ݵ����ʵ�����ϵ�жϲ��뷴Ӧ�����ӵķ�Ӧ�̶ȣ�������д���ӷ���ʽ��
��� �⣺��1�����������������ȫ���������ӵĻ�����Ϊ�ᣬ�������������ΪHClO4��
�ʴ�Ϊ��HClO4��
��2���ٴ���ΪNa2CO3�����ɽ������Ӻ�������ӹ��ɵģ������Σ����Ǽ�ʴ�Ϊ�����
�ڻ����Ȳ��ǵ���ʣ��ֲ��Ƿǵ���ʣ�������HCl��ˮ��Һ���Ȳ��ǵ����Ҳ���Ƿǵ���ʣ�
�ʴ�Ϊ�����ᡢ���
��3��̼����������Һ����ȫ�������������̼��������ӣ�����뷽��ʽΪ��NaHCO3�TNa++HCO3-��������ˮ��Һ����ȫ����������Ӻ���������ӣ����뷽��ʽΪ H2SO4�T2H++SO42-���ʴ�Ϊ��NaHCO3�TNa++HCO3-��H2SO4�T2H++SO42-��
��4��KHSO4�� NaHCO3���ɽ��������Ӻ���ʽ�����ӹ��ɣ���������ʽ�Σ����Խ����Ƿ�Ϊһ�࣬KHSO4��H2SO4������ˮ��Һ�е����H+��ˮ��Һ�����ԣ�����������KHSO4��H2SO4��Ϊͬһ��𣬹ʴ�Ϊ��KHSO4�� NaHCO3���ɽ��������Ӻ���ʽ�����ӹ��ɣ���������ʽ�Σ�KHSO4��H2SO4������ˮ��Һ�е����H+��ˮ��Һ�����ԣ�
��5����NaHSO4�����ʵ���Ϊ2mol������2molH+���ӣ�2molSO42-���ӣ�Ba��OH��2�����ʵ���Ϊ1mol������1molBa2+���ӣ�2molOH-���ӣ�
��Ӧ�����ӷ���ʽΪ2H++SO42-+2OH-+Ba2+=BaSO4��+2H2O����NaHSO4�����ʵ���Ϊ1mol������1molH+���ӣ�1molSO42-���ӣ�Ba��OH��2�����ʵ���Ϊ2mol������2molBa2+���ӣ�4molOH-���ӣ���Ӧ�����ӷ���ʽΪH++SO42-+OH-+Ba2+=BaSO4��+H2O���ʴ�Ϊ��2H++SO42-+Ba2++2OH-�TBaSO4��+2H2O��H++SO42-+Ba2++OH-�TBaSO4��+H2O��
���� ���⿼�����ʵķ��ࡢ�����йص����ӷ���ʽ����д����Ϥ�������ʵ���ɺ����ʵó��ķ����������ʵ������ǽ����Ĺؼ�����Ŀ�ѶȲ���

 �߽�������ϵ�д�
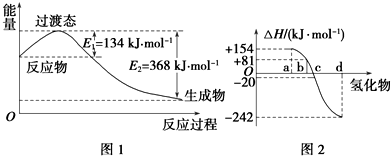
�߽�������ϵ�д���1����ͼ1��ʾ��NO2��CO��Ӧ����CO2��NO�����������仯ʾ��ͼ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ��NO2��g��+CO��g��=CO2��g��+NO��g����H=-234kJ/mol��
��2����ѧ��Ӧ���ʱ��뷴Ӧ���������ļ����йأ�
����֪��H2��g��+Cl2��g���T2HCl��g��
��H=-185kJ•mol-1
����գ�
| ���ۼ� | H-H | Cl-Cl | H-Cl |
| ����/��kJ•mol-1�� | 436 | 247 | 432 |
��ͼ2�б�ʾ����Ԫ���������������������⻯��ʱ���ʱ����ݣ������ʱ����ݿ�ȷ��
a��b��c��d�ֱ��������Ԫ�أ���д��������������ѧ��״̬�£������ֽⷴӦ���Ȼ�ѧ����ʽ��H2Se��g��=Se��s��+H2��g����H=-81kJ/mol��
��3����֪��Fe2O3��s��+3CO��g���T2Fe��s��+3CO2��g��
��H=-25kJ•mol-1��
3Fe2O3��s��+CO��g���T2Fe3O4��s��+CO2��g��
��H=-47kJ•mol-1��
Fe3O4��s��+CO��g���T3FeO��s��+CO2��g��
��H=+19kJ•mol-1��
��д��CO��ԭFeO���Ȼ�ѧ����ʽ��FeO��s��+CO��g���TFe��s��+CO2��g����H=-11kJ/mol��
| A�� | 100mL 2.5mol/L NaCl | B�� | 200mL 2mol/L MgCl2 | ||
| C�� | 500mL 1mol/L AlCl3 | D�� | 200mL 5mol/L KClO3 |
| A�� | 2Na+2NH3=2NaNH2+H2�� | B�� | 3SiH4+4NH3=Si3N4+12H2 | ||
| C�� | NH3+HCl=NH4Cl | D�� | 2NH3+3CuO=3Cu+N2+3H2O |
| A�� | 0.5 mol O3��11.2 L O2�����ķ�����һ����� | |
| B�� | 25����60��ʱ��ˮ��pH��� | |
| C�� | �к͵�����������ʵ���Ũ�ȵ�����ʹ��������ĵ�n��NaOH����� | |
| D�� | 2SO2��g��+O2��g���T2SO3��g����4SO2��g��+2O2��g���T4SO3��g���ġ�H��� |