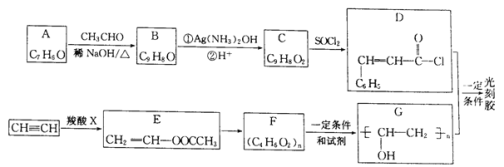
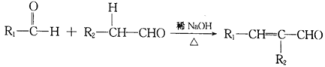
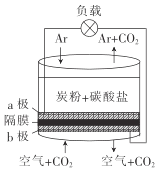
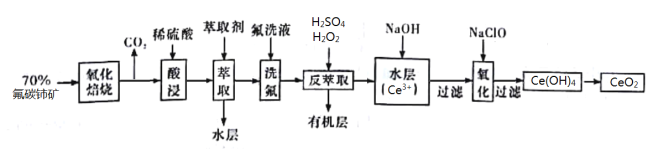
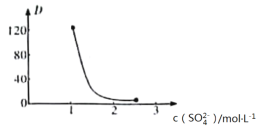
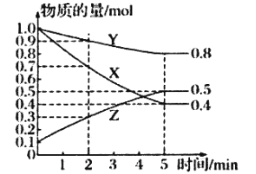
��Ŀ����
����Ŀ����������(Na2FeO4)���к�ǿ�������ԣ���һ�����͵���ɫ��ˮ����������ҵ����������(��Ҫ�ɷ���FeCO3������SiO2)Ϊԭ���Ʊ��������������������£�
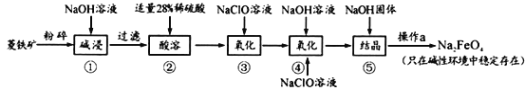
��1��Na2FeO4����Ԫ�صĻ��ϼ�Ϊ_____________��������������ɱ������ʱ�Ļ�ѧ��Ӧ����Ϊ_______________(����������ԭ��Ӧ���������ֽⷴӦ���������Ϸ�Ӧ��)��
��2�������������̣�������м��ʱ�ܷ��ýϱ��˵�Ca(OH)2���NaOH___________ (������������������)��������________________________��
��3��������м���Fe2+ȫ��ת����Fe3+�ķ����ǣ�___________________________��
��4��������г�����Na2FeO4�⣬����NaCl���ɣ������ӷ���ʽΪ_____________����֪���������Na2FeO4��Һ�м��������������ƹ���õ�����Һ�������a������Ϊ_____��
��5�����������У�������ÿ���0.5mol��FeO42-���� NaClO��������Ϊ_______________��
���𰸡�+6 ������ԭ��Ӧ ���� CaSiO3������ˮ���������SiO2 ȡ����������Һ���Թܣ��������� K3Fe(CN)6��Һ��������ɫ�������� Fe2+�Ѿ�ȫ��ת����Fe3+ 2Fe3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O ���� 74.5g
��������
��1������Na2FeO4�л��ϼ۴�����Ϊ�㣬��FeԪ�صĻ��ϼ�Ϊ+6�ۣ��������ƾ��к�ǿ�������ԣ����Ը�����������ɱ������ʱ�Ļ�ѧ��Ӧ����Ϊ������ԭ��Ӧ���𰸣�+6�� ������ԭ��Ӧ��
��2����NaOH�ܽ�SiO2���ɹ��������ܽ���ˮ,�Ӷ���FeCO3�������ȥ�������ýϱ��˵�Ca(OH),���NaOH����Ϊ���ɵ�CaSiO3������ˮ���������SiO2���𰸣����ܣ� CaSiO3������ˮ���������SiO2��
��3��������м���Fe2+ȫ��ת����Fe3t�ķ����ǣ�ȡ����������Һ���Թܣ���������K3Fe(CN)6����Һ������ɫ������������Fe2+�Ѿ�ȫ��ר����Fe3+���𰸣�ȡ����������Һ���Թܣ��������� K3Fe(CN)6��Һ��������ɫ�������ɣ��� Fe2+�Ѿ�ȫ��ת����Fe3+��
��4��������г�����Na2FeO4���NaCl����,�����ӷ�Ӧ����ʽΪ2Fe3++3ClO-+10OH-=2FeO42-+3Cl-+5H2O����֪���������Na2FeO4��Һ�м��������������ƹ���õ�����Һ�����������Һ��Ӧ�ù��������������a������Ϊ���ˡ�
��5�����������У�+2�۵�FeԪ���Ⱥ�NaClO������+3�ۺ�+6�ۣ����������̿������ݵ����غ��֪������+2�������+6�ۣ���ɵù�ϵʽFeO42-~ 2NaClO~4e-����ˣ�ÿ���0.5mol��FeO42-��Ҫ����1.0mol��NaClO��������Ϊ74.5g���𰸣�74.5g��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�����Ŀ����������Żش𣺢�HCl ��NaOH ��Cl2 ��H2O ��NH4Cl ��P4 ��NH3�� H2O ��Na2O2 ��HClO ��CaO HF MgCl2��
(1)ֻ�������Ӽ�����____________________
(2)���ڹ��ۻ��������____________________
(3)�ȴ������ӽ��ִ��ڹ��ۼ�����____________________
����ͬѧ���Ѿ�ѧϰ��ͬλ�ء�ͬϵ�ͬ�������塢ͬ���칹�壬����������Щ�����������г��˼������ʣ��뽫���ʵĺ��������д���±��С�
��![]() ��
��![]() �� ��CH3C(CH3)2CH3��
�� ��CH3C(CH3)2CH3��![]() ��
��
��CH4��CH3CH2CH3�� �ܽ��ʯ��ʯī����뭡����밣���16O��17O��18O�����Ҵ�(CH3CH2OH)�ͼ���(CH3OCH3)��������(O2)�����(O3)��
��� | ͬλ�� | ͬϵ�� | ͬ�������� | ͬ���칹�� |
��� | ________ | ___________ | ________ | _______ |