��Ŀ����
�ס��ҡ���������������֮���������ת����ϵ��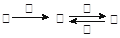
��ش��������⣺
��1������Ϊ���Բ�������ת��Ϊ�ҵ����ӷ���ʽΪ ��
��2������Ϊ�γ��������Ҫ���ʣ���Ļ�ѧʽ ���������ȵ�NaOH��Һ��Ӧ��������Ԫ�ص����̬Ϊ+4��д���÷�Ӧ�����ӷ���ʽΪ��
��
��3�������к���Ŀǰ����ʹ����㷺�Ľ���Ԫ�أ�����ת���ɱ�Ϊ���Ϸ�Ӧ������Һ���������յõ��������� ����ȥ����Һ�������ҵķ����ǣ� ���û�ѧ����ʽ��ʾ������μ�������Һ�еı��������ʵ�鷽�� ��
��Al3+��3AlO2-��6H2O=4Al(OH)3��
��H2S 3S��6OH-=2S2-��SO32-��H2O
��Fe2O3 2FeCl3��Fe= 3FeCl2 ȡ�����ң�FeCl3����Һ���Թ��У��μ����Ը��������Һ�������������Һ��ɫ��������Һ�к��б�FeCl2��
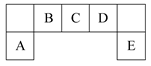
���������������1������Ϊ���Բ��������ΪAl(OH)3����ΪAl3+����ΪAlO2-����ΪOH-����2������Ϊ�γ��������Ҫ���ʣ����ΪH2S����ΪS����ΪSO2����ΪO2����3�������к���Ŀǰ����ʹ����㷺�Ľ���Ԫ�أ���Ϊ������Ԫ�ء���ΪFe,��ΪFeCl3����ΪFeCl2 ����ΪCl2��
���㣺������������й�֪ʶ��

 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д����й���F��Cl��Br��I�ıȽϣ�˵����ȷ����
| A��ԭ�������ĵ�������˵���������Ӷ����� |
| B���������ӵĻ�ԭ����˵���������Ӷ���ǿ |
| C���⻯����ȶ�����˵���������Ӷ���ǿ |
| D���ǽ�������˵���������Ӷ���ǿ |
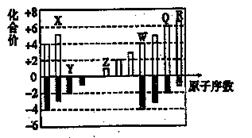
���ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ����±���
| Ԫ�ش��� | X | Y | Z | W |
| ԭ�Ӱ뾶/pm | 160 | 143 | 70 | 66 |
| ��Ҫ���ϼ� | +2 | +3 | +3��+5��-3 | -2 |
A��X��YԪ�صĽ����� X<Y
B��һ�������£�Z������W�ij�������ֱ������ZW2
C��Y������������Ӧ��ˮ����������ϡ��ˮ
D��һ�������£�W���ʿ��Խ�Z���ʴ����⻯�����û�����