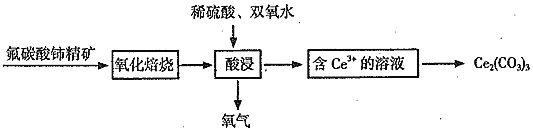
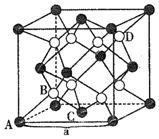
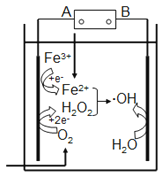
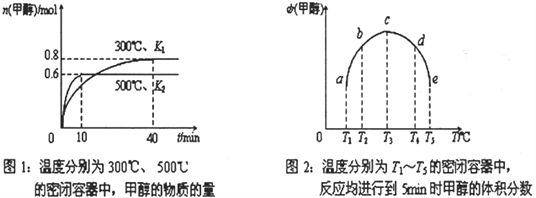
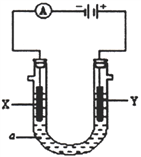
ЬтФПФкШн
ЁОЬтФПЁПГЃЮТЯТЃЌЯђ1L0.1mol/LH2AШмвКжаж№ЕЮМгШыЕШХЈЖШNaOHШмвКЃЌЫљЕУШмвКжаКЌAдЊЫиЕФЮЂСЃЕФЮяжЪЕФСПЗжЪ§гыШмвКpHЕФЙиЯЕШчЭМЃЌЯТСаЫЕЗЈжае§ШЗЕФЪЧЃЈ ЃЉ
A.H2AЕФЕчРыЗНГЬЪНЮЊH2A=HA-+H+ HA-A2-+H+
B.ЪвЮТЯТЃЌNa2AЫЎНтЦНКтГЃЪ§Kh=10-11
C.0.1mol/LNaHAШмвКжаДцдкc(A2-)+c(HA-)ЃМ0.1mol/L
D.ГЃЮТЯТЃЌЕШЮяжЪЕФСПХЈЖШNaHAгыNa2AШмвКЕШЬхЛ§ЛьКЯКѓШмвКЕФpH=3.0
ЁОД№АИЁПB
ЁОНтЮіЁП
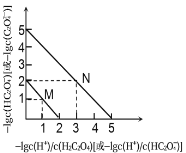
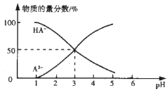
![]() гЩЭМЯѓПЩжЊЃЌpH=1ЕФШмвКжаКЌгаAдЊЫиЕФЮЂСЃЃКВЛКЌH2AКЌгаA2-ЁЂHA-ЃЌЫљвд
гЩЭМЯѓПЩжЊЃЌpH=1ЕФШмвКжаКЌгаAдЊЫиЕФЮЂСЃЃКВЛКЌH2AКЌгаA2-ЁЂHA-ЃЌЫљвд![]() ШмвКжаШЋВПЕчРыЮЊ
ШмвКжаШЋВПЕчРыЮЊ![]() ЃЌЫЕУїЕквЛВНЕчРыЮЊЭъШЋЕчРыЃЌЫљвд
ЃЌЫЕУїЕквЛВНЕчРыЮЊЭъШЋЕчРыЃЌЫљвд![]() ЕФЕчРыЗНГЬЪНЮЊЃК
ЕФЕчРыЗНГЬЪНЮЊЃК![]() ЃЌ
ЃЌ![]() ЃЌЙЪAДэЮѓЃЛ
ЃЌЙЪAДэЮѓЃЛ
BЃЎ![]() ЪБ
ЪБ![]() ЃЌдђ
ЃЌдђ![]() ЫЎНтЦНКтГЃЪ§
ЫЎНтЦНКтГЃЪ§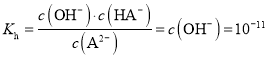 ЃЌЙЪBе§ШЗЃЛ
ЃЌЙЪBе§ШЗЃЛ
CЃЎ![]() ШмвКжаШЋВПЕчРыЃЌВЛДцдк
ШмвКжаШЋВПЕчРыЃЌВЛДцдк![]() ЗжзгЃЌдђгЩЮяСЯЪиКуПЩжЊЃЌ
ЗжзгЃЌдђгЩЮяСЯЪиКуПЩжЊЃЌ![]() ЃЌЙЪCДэЮѓЃЛ
ЃЌЙЪCДэЮѓЃЛ
DЃЎГЃЮТЯТЃЌcЃЈA2-ЃЉ=cЃЈHA-ЃЉЪБЃЌKa=![]() =cЃЈH+ЃЉ=10-3ЃЌдђA2-ЫЎНтЦНКтГЃЪ§Kh=
=cЃЈH+ЃЉ=10-3ЃЌдђA2-ЫЎНтЦНКтГЃЪ§Kh=![]() =10-11ЃМKaЃЌдђЕШЮяжЪЕФСПХЈЖШЕФNaHAгыNa2AШмвКЕШЬхЛ§ЛьКЯКѓЃЌA2-ЫЎНтГЬЖШаЁгкHA-ЕчРыГЬЖШЃЌЕМжТШмвКжаcЃЈA2-ЃЉЃОcЃЈHA-ЃЉЃЌдђcЃЈH+ЃЉЃМ10-3ЃЌШмвКЕФpHЃО3ЃЌЙЪDДэЮѓЃЛ
=10-11ЃМKaЃЌдђЕШЮяжЪЕФСПХЈЖШЕФNaHAгыNa2AШмвКЕШЬхЛ§ЛьКЯКѓЃЌA2-ЫЎНтГЬЖШаЁгкHA-ЕчРыГЬЖШЃЌЕМжТШмвКжаcЃЈA2-ЃЉЃОcЃЈHA-ЃЉЃЌдђcЃЈH+ЃЉЃМ10-3ЃЌШмвКЕФpHЃО3ЃЌЙЪDДэЮѓЃЛ
Д№АИбЁBЁЃ

ЁОЬтФПЁПЪЕбщЪвжЦБИ1ЃЌ2-ЖўфхввЭщЕФЗДгІжаПЩФмДцдкЕФжївЊИБЗДгІгаЃКввДМдкХЈСђЫсЕФДцдкЯТдкl40ЁцЭбЫЎЩњГЩввУбЁЃгУЩйСПЕФфхКЭзуСПЕФввДМжЦБИ1ЃЌ2ЁЊЖўфхввЭщЕФзАжУШчЯТЭМЫљЪОЃК

гаЙиЪ§ОнСаБэШчЯТЃК
ввДМ | 1ЃЌ2-ЖўфхввЭщ | ввУб | |
зДЬЌ | ЮоЩЋвКЬх | ЮоЩЋвКЬх | ЮоЩЋвКЬх |
УмЖШЃЏgЁЄcm-3 | 0.79 | 2.2 | 0.71 |
ЗаЕуЃЏЁц | 78.5 | 132 | 34.6 |
ШлЕуЃЏЁц | вЛl30 | 9 | -1l6 |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉдкДЫЪЕбщжаЃЌЗДгІЗНГЬЪНЮЊЃК_________ЃЛ________ЁЃ
ЃЈ2ЃЉвЊОЁПЩФмбИЫйЕиАбЗДгІЮТЖШЬсИпЕН170ЁцзѓгвЃЌЦфзюжївЊФПЕФЪЧ_____ЃЛ(Ьюе§ШЗбЁЯюЧАЕФзжФИ)
aЃЎв§ЗЂЗДгІ bЃЎМгПьЗДгІЫйЖШ cЃЎЗРжЙввДМЛгЗЂ dЃЎМѕЩйИБВњЮяввУбЩњГЩ
ЃЈ3ЃЉдкзАжУCжагІМгШы_______ЃЌЦфФПЕФЪЧЮќЪеЗДгІжаПЩФмЩњГЩЕФЫсадЦјЬхЃК(Ьюе§ШЗбЁЯюЧАЕФзжФИ)
aЃЎЫЎ bЃЎХЈСђЫс cЃЎЧтбѕЛЏФЦШмвК dЃЎБЅКЭЬМЫсЧтФЦШмвК
ЃЈ4ЃЉШєВњЮяжагаЩйСПЮДЗДгІЕФBr2ЃЌзюКУгУ_________ЯДЕгГ§ШЅЃЛ(Ьюе§ШЗбЁЯюЧАЕФзжФИ)
aЃЎЫЎ bЃЎЧтбѕЛЏФЦШмвК cЃЎЕтЛЏФЦШмвК dЃЎввДМ
ЃЈ5ЃЉХаЖЯИУжЦБИЗДгІвбОНсЪјЕФзюМђЕЅЗНЗЈЪЧ_____________ЃЛ
ЃЈ6ЃЉЗДгІЙ§ГЬжагІгУРфЫЎРфШДзАжУDЃЌЦфжївЊФПЕФЪЧ________________ЃЛЕЋгжВЛФмЙ§ЖШРфШД(ШчгУБљЫЎ)ЃЌЦфдвђЪЧ_____________________ЁЃ