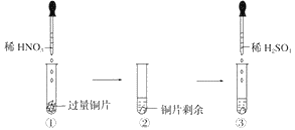
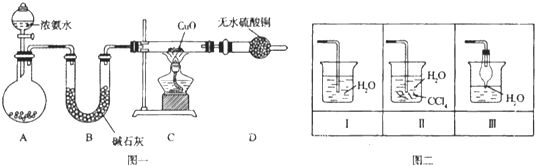
��Ŀ����
����Ŀ���������ȣ�ClO2���ǹ��ʹ��ϸ�Ч����ȫ��ɱ�������ʼ��������Ƽ����������Ʒ����ҵ���Ʊ�ClO2�ķ����ܶ࣬NaClO3 ��NaClO2����ȡClO2�ij���ԭ�ϡ����������գ�
��1�����·�Ӧ���Ʊ�ClO2��һ�ַ�����H2C2O4+2NaClO3+H2SO4��Na2SO4+2CO2��+2ClO2��+2H2O
������Ӧ�������ڵ�������Ԫ�ص�ԭ�Ӱ뾶��С˳����___������ԭ�Ӱ뾶���Ԫ�ص�ԭ�ӣ����������Ų�ʽΪ___���������___�ֲ�ͬ�����ĵ��ӡ�
��2��ClO2�ķ��ӹ���Ϊ��V���Σ���ClO2��___��ѡ���������������Ǽ����������ӣ�����ˮ�е��ܽ�ȱ�����___��ѡ������������С������һ��������
��3��ClO2����ǿ�����ԣ���ClO2��Cl2������ʱ����������ԭΪCl-��ClO2�����������ǵ�����Cl2��___��������2λС������
��4������NaClO2Ϊԭ����ȡClO2����Ҫ�������___���������������ԭ�����Ե����ʡ�
��5����ҵ����ȡNaClO3ͨ����ⷨ���У����ʱ����ͬ��Ӧ�����µ��ܷ�Ӧ�ֱ�Ϊ��
4NaCl +18H2O��4NaClO3+3O2��+18H2�������Ի�����
NaCl +3H2O��NaClO3 +3H2�������Ի�����
�ٵ��ʱ��������___��������
�ڸ������ڹ�ҵ����NaClO3�ķ�Ӧ������___������__��
���𰸡�Na>S>Cl 1s22s22p63s1 4 ���� �� 2.63 ������ ���� ���Ի��� ת�Ƶ��Ӷ����������ƣ����������ʸߣ�ˮ�����٣���ͬʱ������������������Ը���ȫ
��������
��1����Ӧ�������ڵ������ڵ�Ԫ��ΪNa��S��Cl��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С��ԭ�Ӱ뾶���Ԫ��ΪNa����ϵ����Ų�ʽ1s22s22p63s1���
��2��ClO2���ӹ���Ϊ��V���Σ�����������IJ��ص���Ϊ���Է��ӣ�������ˮ��
��3��1molCl2���Ի��2mol���ӣ�1molClO2���Ի�õ���5mol���ӣ�
��4����NaClO2Ϊԭ����ȡClO2��ClԪ�ػ��ϼ���+3�����ߵ�+4�ۣ�
��5���ٵ��ʱ��������ˮ��ԭ���ɣ�
�ڹ�ҵ����ʱ��Ӧע�ⰲȫ���⡣
��1����Ӧ�������ڵ������ڵ�Ԫ��ΪNa��S��Cl��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С����ԭ�Ӱ뾶��С˳��ΪNa��S��Cl��ԭ�Ӱ뾶���Ԫ��ΪNa����ϵ����Ų�ʽ1s22s22p63s1��ͬһ�������������ͬ����������������ͬ�ĵ��ӣ��ʴ�Ϊ��Na��S��Cl��1s22s22p63s1��4��
��2��ClO2���ӹ���Ϊ��V���Σ�����������IJ��ص���Ϊ���Է��ӣ�������ˮ������ˮ�е��ܽ�ȱ������ʴ�Ϊ�����ԣ���
��3������������71g�������õ��ĵ�����Ϊ��![]() ��ClO2�õ��ĵ�����Ϊ��
��ClO2�õ��ĵ�����Ϊ��![]() ����ClO2������Ч����Cl2�ı���Ϊ
����ClO2������Ч����Cl2�ı���Ϊ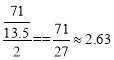 ���ʴ�Ϊ��2.63��
���ʴ�Ϊ��2.63��
��4����NaClO2Ϊԭ����ȡClO2��ClԪ�ػ��ϼ���+3�����ߵ�+4�ۣ�Ӧ�������������ʴ�Ϊ��������
��5���ٵ��ʱ��������ˮ��ԭ���ɣ���Ӧ���������ɣ��ʴ�Ϊ������
���ɵ�ⷽ��ʽ��֪���Ի�����������Ϊ�������������Ի������������������������±�ը��Σ�գ������Ի������������ʸߣ�ˮ�����٣��ڹʴ�Ϊ�����Ի�����ת�Ƶ��Ӷ����������ƣ����������ʸߣ�ˮ�����٣���ͬʱ������������������Ը���ȫ��

 һ����������ϵ�д�
һ����������ϵ�д�����Ŀ����ѧ��Ӧ���ʺ���������������������ء�
��1��ijѧ��Ϊ��̽��п�����ᷴӦ�����е����ʱ仯����400 mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų���������ʵ���¼���£��ۼ�ֵ����
ʱ��/min | 1 | 2 | 3 | 4 | 5 |
�������/mL����״���� | 100 | 240 | 464 | 576 | 620 |
����һʱ��η�Ӧ�������________min������0��1������1��2������2��3������3��4������4��5������ԭ����_____��
����3��4 minʱ����������Ũ�ȱ仯����ʾ�ĸ÷�Ӧ����______������Һ������䣩��
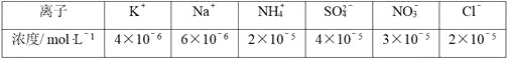
��2����һѧ��Ϊ���Ʒ�Ӧ���ʷ�ֹ��Ӧ�������Բ�������������������������м���������������Һ�Լ�����Ӧ���ʣ�����Ϊ�����е���________������ĸ����
A.����ˮ B.KCl��Һ
C. KNO3��ҺD.Na2SO4��Һ
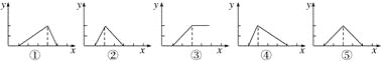
��3��ij�¶�����4 L�ܱ������У�X��Y��Z������̬���ʵ����ʵ�����ʱ��仯������ͼ��
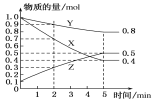
�ٸ÷�Ӧ�Ļ�ѧ����ʽ��_________��
�ڸ÷�Ӧ�ﵽƽ��״̬�ı�־��____������ĸ����
A.Y����������ڻ�������б��ֲ���
B.X��Y�ķ�Ӧ���ʱ�Ϊ3��1
C.����������ѹǿ���ֲ���
D.��������������������ֲ���
E.����1 mol Y��ͬʱ����2 mol Z