��Ŀ����
���������ҹ��ܶ�����������ص������������������PM2.5��300���ϣ�PM2.5��ָ������ֱ��С�ڻ����2.5�Ŀ���������к���Ǧ���������Ԫ�ض��������к���PM2.5����Դ����ȼ�ͻ�����β��������ʩ���ﳾ���������ա��ոѷ���ֱ���ŷŵ�ϸ�����Ҳ�п����ж�������������ͻӷ����л���������ӵĻ�ѧ��Ӧת�����ɵĶ���ϸ��������ش��������⣺
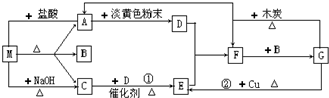
��1���������ڱ��е�λ���ǣ� ��
��2���������⻯���н��ȶ����ǣ� �����⻯�����ʽ����
��3�����ж��鼰�仯�������ʵ�Ԥ���д������ ��
A�������������ȼ�� B�������Mg��Ӧ
C�����������鲻����NaOH��Һ��Ӧ D����������Ա�������
��4��CO2Ҳ����������ЧӦ��Ӱ�컷����CO2���� ���壬д����ṹʽ�� ��C��N��OԪ��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ�� ���ȽϷе�ߵͣ�SO2CO2�����������������=������
��5��Ϊ�˷������������Dz�ȡ�˶��ִ�ʩ�����м����к����ʵ��ŷ�����Ҫ��ʩ֮һ��Ϊ����SO2���ŷţ���ҵ����ʱ�ü�Һ���մ���SO2��д��SO2�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� ��Ϊ��������β���еĵ���������ŷţ��������������������м���Ĵ�����ʹNO��COת��Ϊ�����������壬д���÷�Ӧ�ķ���ʽ�� ��
��1���������ڱ��е�λ���ǣ�
��2���������⻯���н��ȶ����ǣ�
��3�����ж��鼰�仯�������ʵ�Ԥ���д������
A�������������ȼ�� B�������Mg��Ӧ
C�����������鲻����NaOH��Һ��Ӧ D����������Ա�������
��4��CO2Ҳ����������ЧӦ��Ӱ�컷����CO2����
��5��Ϊ�˷������������Dz�ȡ�˶��ִ�ʩ�����м����к����ʵ��ŷ�����Ҫ��ʩ֮һ��Ϊ����SO2���ŷţ���ҵ����ʱ�ü�Һ���մ���SO2��д��SO2�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��
���㣺���������������Ⱦ������,Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��,��ѧӦ��
��������1�����������ڵ���Ԫ�ط��������ڱ��е�λ�ã�
��2��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���
��3������ͬ����Ԫ�ص����ʾ��������Ժ͵ݱ�����������
��4��CO2�ɷ��ӹ��ɣ����ڷ��Ӿ��壻������̼Ϊֱ���ͽṹ�������д�������̼��˫����ͬ����Ԫ���������ԭ�Ӱ뾶��С��ͬ�����͵ķ��Ӿ�����Է�������Խ���۷е�Խ�ߣ�
��5���������ƹ���������������������Ʒ�Ӧ�����������ƺ�ˮ��CO��NO��Ӧ������������Ϊ�����Ͷ�����̼��
��2��Ԫ�صķǽ�����Խǿ����Ӧ���⻯��Խ�ȶ���
��3������ͬ����Ԫ�ص����ʾ��������Ժ͵ݱ�����������
��4��CO2�ɷ��ӹ��ɣ����ڷ��Ӿ��壻������̼Ϊֱ���ͽṹ�������д�������̼��˫����ͬ����Ԫ���������ԭ�Ӱ뾶��С��ͬ�����͵ķ��Ӿ�����Է�������Խ���۷е�Խ�ߣ�
��5���������ƹ���������������������Ʒ�Ӧ�����������ƺ�ˮ��CO��NO��Ӧ������������Ϊ�����Ͷ�����̼��
���
�⣺��1�������ڵ���Ԫ�أ���Ԫ�����ڱ��е������ڵ�VA�壻
�ʴ�Ϊ���������ڵ�VA�壻
��2���ǽ����ԣ��飼�������⻯����ȶ��ԣ�As3H��H2S��
�ʴ�Ϊ��H2S��
��3��A������������ȼ�գ���Ҳ����������ȼ�գ�����ȷ��
B����������Mg��Ӧ�������Mg��Ӧ������ȷ��
C����������������NaOH��Һ��Ӧ����������������NaOH��Һ��Ӧ���ʴ���
D���ǽ����ԣ��ף��飬������������Ա�������������ȷ��
��ѡC��
��4��CO2���ڷ��Ӿ��壻������̼Ϊֱ���ͽṹ��������̼�ĽṹʽΪ��O=C=O��ͬ����Ԫ���������ԭ�Ӱ뾶��С�����뾶��C��N��O��SO2��CO2�Ƿ��Ӿ��壬��Է�������Խ���۷е�Խ�ߣ����Էе�ߵͣ�SO2 ��CO2��
�ʴ�Ϊ�����ӣ�O=C=O��C��N��O������
��5��SO2�����NaOH��Һ��Ӧ����NaOH�����������ɵ������Σ���ѧ����ʽΪ��SO2+2NaOH=Na2SO3+H2O��ʹCO��NO��Ӧ������������Ϊ�����Ͷ�����̼���÷�ӦΪ2NO+2CO
N2+2CO2��
�ʴ�Ϊ��SO2+2NaOH�TNa2SO3+H2O��2NO+2CO
N2+2CO2��
�ʴ�Ϊ���������ڵ�VA�壻
��2���ǽ����ԣ��飼�������⻯����ȶ��ԣ�As3H��H2S��
�ʴ�Ϊ��H2S��
��3��A������������ȼ�գ���Ҳ����������ȼ�գ�����ȷ��
B����������Mg��Ӧ�������Mg��Ӧ������ȷ��
C����������������NaOH��Һ��Ӧ����������������NaOH��Һ��Ӧ���ʴ���
D���ǽ����ԣ��ף��飬������������Ա�������������ȷ��
��ѡC��
��4��CO2���ڷ��Ӿ��壻������̼Ϊֱ���ͽṹ��������̼�ĽṹʽΪ��O=C=O��ͬ����Ԫ���������ԭ�Ӱ뾶��С�����뾶��C��N��O��SO2��CO2�Ƿ��Ӿ��壬��Է�������Խ���۷е�Խ�ߣ����Էе�ߵͣ�SO2 ��CO2��
�ʴ�Ϊ�����ӣ�O=C=O��C��N��O������
��5��SO2�����NaOH��Һ��Ӧ����NaOH�����������ɵ������Σ���ѧ����ʽΪ��SO2+2NaOH=Na2SO3+H2O��ʹCO��NO��Ӧ������������Ϊ�����Ͷ�����̼���÷�ӦΪ2NO+2CO
| ||
�ʴ�Ϊ��SO2+2NaOH�TNa2SO3+H2O��2NO+2CO
| ||
������������Ҫ������Ԫ�������ɡ���������͡�������Ⱦ��Ĵ������ѶȲ���ע��֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
�����Ŀ
��Na��H2O�ķ�Ӧ�У�������
| A��Na�������� |
| B����Ӧʵ�������û�ˮ�е����� |
| C����Ӧʵ�������û�ˮ�������������H+ |
| D��H2���������� |
���и������������ʵı仯����������ȷ���ǣ�������
| A�����뾶��Al3+��Mg2+��Na+��F- |
| B���ȶ��ԣ�HI��HBr��HCl��HF |
| C�����ԣ�HClO4��H2SO4��H3PO4��H4SiO4 |
| D�����ԣ�Ca��OH��2��KOH��Mg��OH��2��Al��OH��3 |
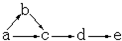 a��b��c��d��e�Ǻ���һ����ͬԪ�ص��������ʣ��ɷ�������ת����
a��b��c��d��e�Ǻ���һ����ͬԪ�ص��������ʣ��ɷ�������ת����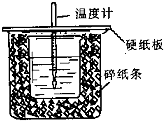
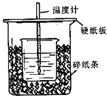 ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��ش��������⣺