��Ŀ����
11��A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬EԪ��ԭ�ӵĺ����������AԪ��ԭ�Ӻ�����������������B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ�������������C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ�������������DԪ��ԭ�������������Ǵ�����������һ�룮�ش��������⣺
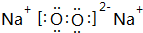
��1���õ���ʽ��ʾB��F��Ԫ���γɻ�����Ĺ��̣�
 ��
����2��A��C��Ԫ�صĻ�������B���������ﷴӦ�����ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
��3��D��̬�⻯����ȶ���С�ڣ�����ڡ�����С�ڡ���E����̬�⻯����ȶ��ԣ�
��4����B��A��ȼ��������ĵ���ʽ
 ��
��
���� A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬��EԪ��ԭ�ӵĺ����������AԪ��ԭ�Ӻ�������������������AΪ��Ԫ�أ�EΪ��Ԫ�أ�DԪ��ԭ�������������Ǵ�����������һ�룬DԪ�ص�ԭ������������Ԫ�أ���D��3�����Ӳ㣬������������Ϊ4����DΪ��Ԫ�أ�B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ���������������B��C����������֮��Ϊ4��ԭ������B��C������Ԫ�أ���B��C��Ԫ�ز�����Ϊͬ��Ԫ�أ�����������Ϊ1��3��ϣ�ԭ������BС��C����BΪ��Ԫ�أ�CΪ��Ԫ�أ�C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ���������������FԪ��ԭ������������Ϊ3+4=7����FΪ��Ԫ�أ�
��� A��B��C��D��E��F���ֶ�����Ԫ�أ�ԭ��������������A��Eͬ���壬��EԪ��ԭ�ӵĺ����������AԪ��ԭ�Ӻ�������������������AΪ��Ԫ�أ�EΪ��Ԫ�أ�DԪ��ԭ�������������Ǵ�����������һ�룬DԪ�ص�ԭ������������Ԫ�أ���D��3�����Ӳ㣬������������Ϊ4����DΪ��Ԫ�أ�B��C��Ԫ��ԭ�ӵ�����������֮�͵���DԪ��ԭ�ӵ���������������B��C����������֮��Ϊ4��ԭ������B��C������Ԫ�أ���B��C��Ԫ�ز�����Ϊͬ��Ԫ�أ�����������Ϊ1��3��ϣ�ԭ������BС��C����BΪ��Ԫ�أ�CΪ��Ԫ�أ�C��D��Ԫ��ԭ������������֮�͵���FԪ��ԭ�ӵ���������������FԪ��ԭ������������Ϊ3+4=7����FΪ��Ԫ�أ�
��1��BΪ��Ԫ�أ�FΪ��Ԫ�أ������γ��Ȼ��ƣ����������������ӹ��ɣ��õ���ʽ��ʾ�Ȼ����γɻ�����Ĺ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��2��A��C��Ԫ�صĻ�����Ϊ��������B����������Ϊ�������ƣ����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ�����ӷ���ʽΪ��Al2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��3��DΪ��Ԫ�أ�EΪ��Ԫ�أ��ǽ�����Si��S���ǽ�����Խǿ���⻯��Խ�ȶ���D��̬�⻯����ȶ���С��E����̬�⻯����ȶ��ԣ�
�ʴ�Ϊ��С�ڣ�
��4����A��ȼ������Na2O2������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����ʽṹ��λ�ù�ϵӦ�ã��ƶ�Ԫ���ǹؼ���������ú�������Ų����ɼ��ṹ��λ�ù�ϵ�����ƶϣ��Ƕ�ѧ���ۺ������Ŀ��飮

| A�� | ���ȷ�������Fe2O3+2Al$\frac{\underline{\;\;��\;\;}}{\;}$Al2O3+2Fe | |
| B�� | ��ҵ����NH3�Ʊ�NO��4NH3+5O2$\frac{\underline{\;\;��\;\;}}{\;}$4NO+6H2O | |
| C�� | ��������Ӱ��ĺ���������CaCO3��ĩ��CO32-+2H+$\frac{\underline{\;����\;}}{\;}$H2O+CO2�� | |
| D�� | �����������ں����������Ϊ������2Na2O2+2CO2�T2Na2CO3+O2 |
| A�� | NaCl | B�� | LiCl | C�� | MgO | D�� | Na2S |
| A�� | R�����������ΪRO3 | B�� | Rһ���ǵڢ�A��Ԫ�� | ||
| C�� | R����̬�⻯����ȼ�� | D�� | R����̬�⻯��������ˮ�Լ��� |
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶 | 0.186 | 0.143 | 0.089 | 0.102 | 0.074 |
| ��Ҫ���ϼ� | +1 | +3 | +2 | +6��-2 | -2 |
| A�� | �����ԣ�C��A | B�� | �⻯����ȶ��ԣ�H2D��H2E | ||
| C�� | ������ϡ���ᷴӦ�����ʣ�A��B | D�� | ���ʵ��۵㣺A��B��E |
| A�� | CH3CH2Cl | B�� | CH2Cl-CH2Cl | C�� | CH3-CH2OH | D�� | CH3-COOH |

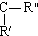 $\stackrel{KMnO4/H+}{��}$RCOOH+
$\stackrel{KMnO4/H+}{��}$RCOOH+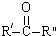
 +H2O
+H2O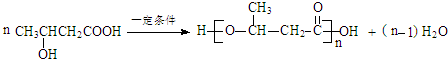 ��
�� ��
��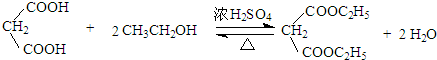 ��
��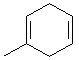 ��
��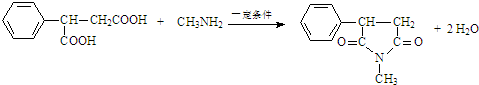 ��
��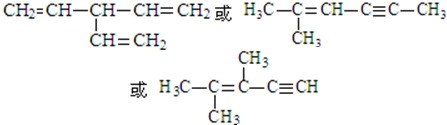 ��д��һ�ּ��ɣ���
��д��һ�ּ��ɣ���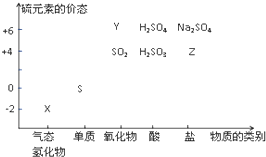 ���ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ�������Ҫ�ӽǣ����仯�������̬�仯Ϊ����Ķ�άת����ϵ��ͼ��ʾ
���ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ�������Ҫ�ӽǣ����仯�������̬�仯Ϊ����Ķ�άת����ϵ��ͼ��ʾ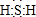 ����ˮ��Һ�ڿ����з����ױ���ǣ�д����Ӧ�Ļ�ѧ����ʽ2H2S+O2=2S��+2H2O��
����ˮ��Һ�ڿ����з����ױ���ǣ�д����Ӧ�Ļ�ѧ����ʽ2H2S+O2=2S��+2H2O�� �������в��ϼ���ˮ�����������ˮ���Ĺ�ϵ��ͼ����ش��й�a��b��c�������ʾ�����⣺
�������в��ϼ���ˮ�����������ˮ���Ĺ�ϵ��ͼ����ش��й�a��b��c�������ʾ�����⣺