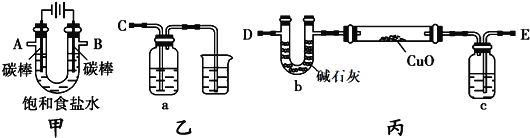
��Ŀ����
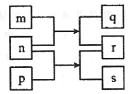
����Ŀ������������Ԫ��W��X��Y��Z��ԭ�������������ӣ�m��n��p������ЩԪ����ɵĶ�Ԫ�����r��һ����̬���ʣ�nΪ����ɫ��ĩ���������ת����ϵ��ͼ��ʾ�������£�0. 0lmol/L��s��ҺpHΪ12��X����������W��Z��������֮�͵�һ�롣����˵����ȷ����
A.ԭ�Ӱ뾶��W<X<Y
B.���⻯��е㣺Z<X<Y
C.n��s�о��������Ӽ����ۼ�
D.q����ˮʱ�¶����ߣ�֤����ˮ����̷���
���𰸡�C
��������
n��һ�ֵ���ɫ��ĩ������p��Ӧ����s��r����0.01molL-1��s��Һ��pHΪ12��sΪһԪǿ�rΪY�����嵥�ʣ���sΪNaOH��nΪNa2O2��pΪH2O��rΪO2������֪mΪCO2��qΪNa2CO3�����ԭ��������֪WΪH��XΪC��YΪO��ZΪNa���ݴ˷������
A��Hԭ�Ӻ���ֻ��1�����Ӳ㣬C��O�������2�����Ӳ㣬ͬ����Ԫ�غ˵����Խ��ԭ�Ӱ뾶ԽС�����ԭ�Ӱ뾶��W<Y<X����A����
B��C��Ӧ���⻯��ΪCH4��O��Ӧ���⻯��ΪH2O��Na��Ӧ���⻯��ΪNaH��CH4��H2O��Ϊ���Ӿ��壬NaHΪ���Ӿ��壬H2O����֮���ܹ��γ��������˼��⻯��е㣺X<Y<Z����B����
C��Na2O2��NaOH����Na+���ԭ�ӵ���������ɵ����ӻ����Na+��������֮��������Ӽ����������ڴ��ڹ��ۼ�����C��ȷ��
D��Na2CO3����ˮʱ��Na+��CO32-���γ�ˮ������ʱ����ȣ�������ˮ����ȣ�ˮ������������ȷ�Ӧ����D����
�ʴ�Ϊ��C��

 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ��������ڱ��е�λ�ã��ش�����
�� ���� | IA | IIA | IIIA | IVA | VA | VIA | VIIA |
1 | �� | ||||||
2 | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� |
(1)���Т������ڰ뵼����ϵ�Ԫ�أ���Ԫ�ط���Ϊ____���������ڱ��е�λ����____��
(2)�ڡ��ۡ��ܵ�ԭ�Ӱ뾶��С��____(��Ԫ�ط���)����ԭ�ӽṹʾ��ͼ��____��
(3)�ݡ��ޡ��ߵ�����������Ӧ��ˮ���������ǿ����____(�ѧʽ)��
(4)�ٺ͢��γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�����ĵ���ʽΪ____��
(5)�ݺ͢��γɵĻ���������____���������ӻ��������������ۻ������������õ���ʽ��ʾ���γɹ���____