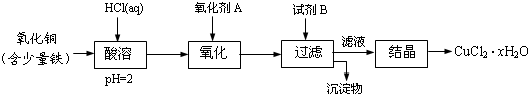
��Ŀ����
8��һ���¶��£���2L���ܱ������г���2molPCl3��g����1molCl2��g����������Ӧ��PCl3��g��+Cl2��g��?PCl5��g����5min��ƽ�⣬PCl3��g����ת����Ϊ20%�����ҷų�37.2kJ����������ش��������⣺��1��ǰ5min�ڣ�v��Cl2��=0.04mol•L-1•min�����¶��£�ƽ�ⳣ��K=0.83��
��2�������ĸ�ͼ������ȷ˵��������Ӧ�ڽ��е�t1ʱ��ʱ���ﵽƽ��״̬AB��
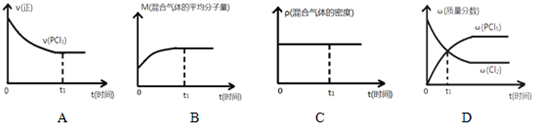
��3�������Ϻ����������ɾ�����������PCl3��g����ת���ʣ�20%���������������=������ƽ�ⳣ��K��С���������С�����䡱����
��4��PCl5��������ˮ����ȫˮ�����������ᣬ�÷�Ӧ�Ļ�ѧ����ʽ��PCl5+4H2O=H3PO4+5HCl��
��5����֪��H3PO4�ĵ���ƽ�ⳣ����Ka1=7.52��10-3��Ka2=6.23��10-8��Ka3=2.2��10-13��H2CO3�ĵ���ƽ�ⳣ����Ka1=4.3��10-7��Ka2=5.61��10-11����H3PO4����Һ��μ��뵽������Na2CO3����Һ�У���д�����ܷ�����Ӧ�����ӷ���ʽH3PO4+2CO32-=HPO42-+2HCO3-��
���� ��1����ƽ��ʱ�μӷ�Ӧ������Ϊxmol����
PCl3��g��+Cl2��g��?PCl5��g��
��ʼ����mol����2 1 0
�仯����mol����x x x
ƽ������mol����2-x 1-x x
����PCl3��g����ת�����з��̼���x���ٸ���v=$\frac{��c}{��t}$����v��Cl2����ƽ�ⳣ��K=$\frac{c��PC{l}_{5}��}{c��PC{l}_{3}����c��C{l}_{2}��}$��
��2��A���淴Ӧ���У�����Ӧ���ʼ��ͣ���Ӧ���ʲ��ٱ仯��˵������ƽ�⣻
B������������������䣬�淴Ӧ���������ʵ�����С��ƽ����Է���������С�������淴Ӧ����ƽ����Է�������������
C������������������䣬�������ݻ����䣬�ܶ�ʼ�ղ��䣻
D��t1ʱ�̺�PCl5��������������Cl2����������С��˵��t1ʱ��ʱ��Ӧ������Ӧ���У�
��3�������Ϻ����������ɾ�������������ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ԭƽ�����ƽ�������ƶ���PCl3��g����ת���ʼ�С��ƽ�ⳣ��K��С��
��4��PCl5��������ˮ����ȫˮ�����������ᣬ������H3PO4��HCl��
��5�������ᡢ̼��ĵ���ƽ�ⳣ����֪������H3PO4��H2CO3��H2PO4-��HCO3-��HPO42-��̼���������������ᷴӦ����̼�����ơ�Na2HPO4��
��� �⣺��1����ƽ��ʱ�μӷ�Ӧ������Ϊxmol����
PCl3��g��+Cl2��g��?PCl5��g��
��ʼ����mol����2 1 0
�仯����mol����x x x
ƽ������mol����2-x 1-x x
PCl3��g����ת����Ϊ20%����$\frac{x}{2}$=20%�����x=0.4��
��v��Cl2��=$\frac{\frac{0.4mol}{2L}}{5min}$=0.04mol/��L��min����ƽ�ⳣ��K=$\frac{c��PC{l}_{5}��}{c��PC{l}_{3}����c��C{l}_{2}��}$=$\frac{\frac{0.4}{2}}{\frac{1.6}{2}��\frac{0.6}{2}}$=0.83��
�ʴ�Ϊ��0.04��0.83��
��2��A���淴Ӧ���У�����Ӧ���ʼ��ͣ���Ӧ���ʲ��ٱ仯��˵������ƽ�⣬��A��ȷ��
B������������������䣬�淴Ӧ���������ʵ�����С��ƽ����Է���������С�������淴Ӧ����ƽ����Է�������������ƽ����Է�����������˵������ƽ�⣬��B��ȷ��
C������������������䣬�������ݻ����䣬�ܶ�ʼ�ղ��䣬��C����
D��t1ʱ�̺�PCl5��������������Cl2����������С��˵��t1ʱ��ʱ��Ӧ������Ӧ���У���D����
��ѡ��AB��
��3�������Ϻ����������ɾ�������������ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ԭƽ�����ƽ�������ƶ���PCl3��g����ת���ʼ�С����PCl3ת���ʣ�20%��ƽ�ⳣ��K��С��
�ʴ�Ϊ��������С��
��4��PCl5��������ˮ����ȫˮ�����������ᣬ������H3PO4��HCl����Ӧ����ʽΪ��PCl5+4H2O=H3PO4+5HCl��
�ʴ�Ϊ��PCl5+4H2O=H3PO4+5HCl��
��5�������ᡢ̼��ĵ���ƽ�ⳣ����֪������H3PO4��H2CO3��H2PO4-��HCO3-��HPO42-��̼���������������ᷴӦ����̼�����ơ�Na2HPO4����Ӧ���ӷ���ʽΪ��H3PO4+2CO32-=HPO42-+2HCO3-��
�ʴ�Ϊ��H3PO4+2CO32-=HPO42-+2HCO3-��
���� ���⿼�黯ѧƽ����㡢��ѧƽ��״̬�жϡ�ƽ�ⳣ��������ƽ�ⳣ��Ӧ�õȣ���5���йؼ��Ǹ��ݵ���ƽ�ⳣ���ж�����ǿ�����Ѷ��еȣ�

| A�� | ���������Ʊ�������ˮ�� | |
| B�� | Ũ����ʢ������ɫƿ�� | |
| C�� | �������ױ�����ú���� | |
| D�� | ����������Һʢ�����������Լ�ƿ�� |
| A�� | ʵ���ҿ���ͨ����ȡ����Һ�ķ�����I2����CCl4��Һ�з������ | |
| B�� | ����Һ�������Ȼ��ƾ���ij��÷���������Ũ������ȴ�ᾧ | |
| C�� | �����Ƶ�����������Һ������������������� | |
| D�� | ����490mL 1mol•L-1��CuS04��Һ����500mL������ƿ |
| A�� | ��״���£�22.4 L�����й��ۼ���ĿΪ19NA | |
| B�� | 12.4g�������к��е�P-P������0.1NA | |
| C�� | 2mol SO2��1mol O2�����V2O5���ڵ������£��ܱ������м��ȷ�Ӧ�����������ʵķ���������2NA | |
| D�� | ��NO2��N2O4���ӹ�NA����������״���£������Ϊ22.4 L |
| A�� | ��С������� | B�� | �����¶� | ||
| C�� | ������� | D�� | �����³���HI���� |
| A�� | ���ȣ��۲��Ƿ�������ų� | |
| B�� | �������ᣬ�۲��Ƿ�������ų� | |
| C�� | ����ˮ���BaCl2��Һ���۲����������� | |
| D�� | ������ɫ��Ӧ���۲�����Ƿ�ʻ�ɫ |
| A�� | ����ԭ�ӵ����ԭ������Ϊ12a/b | |
| B�� | Wg����ԭ�ӵ����ʵ���ΪW/aNA mol | |
| C�� | Wg ����ԭ��������������Ϊ 10W/a�� | |
| D�� | ��Ԫ�ص�Ħ������ΪaNA g/mol |