��Ŀ����
2���⡢̼����������Ҫ�ķǽ���Ԫ�أ����ǵ��ʼ��仯�����ڿ�ѧ�о���ҵ����������Ҫ��Ӧ�ã�
��1����������̬�ĵ����Ų���δ�ɶԵ�����������d������ţ�
a��O��b��N c��Cu d��Cr e��C
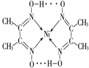
��2�����������W�Ľṹ��ͼ1��ʾ����λ��δ����������ڽṹͼ���á��������W�������ڵ�������λ���ķ���
 ��
����3��C��HԪ���γɵ�ij�л�����������й���16�����ӣ��÷����ЦҼ���м��ĸ�����Ϊ5��1�����л���������һ���������γɵľۺ�����Cԭ�Ӳ�ȡsp3�ӻ���
��4��N��CuԪ���γɵĻ�����ľ����ṹ��ͼ2��ʾ����û�����Ļ�ѧʽΪCu3N���û��������Է�������ΪM��NAΪ�����ӵ����������þ����ı߳�Ϊa pm����þ�����ܶ���$\frac{206}{{a}^{3}{N}_{A}}$g��pm-3��
���� ��1��a��Oԭ����δ�ɶԵ�����2����
b��Nԭ����δ�ɶԵ�����3����
c��Cuԭ����δ�ɶԵ�����1����
d��Crԭ������δ�ɶԵ�����5����
e��Cԭ����δ�ɶԵ�����2����
��2�����йµ��Ӻͺ��пչ����ԭ��֮�������λ������λ���ɺ��йµ��ӶԵ�ԭ��ָ���пչ����ԭ�ӣ�
��3��C��HԪ���γɵ�ij�л�����������й���16�����ӣ�ÿ��̼ԭ�Ӻ���6�����ӣ����Ըû���������ຬ������̼ԭ�ӣ�����4��Hԭ�ӣ�Ϊ��ϩ�����۵���Ϊ�Ҽ�������˫����һ���ǦҼ�һ���Ǧм���
���л���������һ���������γɵľۺ�����Cԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ�������ȷ��ԭ���ӻ���ʽ��
��4���þ�����Nԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=12��$\frac{1}{4}$=3�������仯ѧʽΪCu3N�������ı߳�Ϊa pm�������=a3pm3���ܶ�=$\frac{\frac{M}{{N}_{A}}��1}{V}$��
��� �⣺��1��a��Oԭ����δ�ɶԵ�����2����
b��Nԭ����δ�ɶԵ�����3����
c��Cuԭ����δ�ɶԵ�����1����
d��Crԭ������δ�ɶԵ�����5����
e��Cԭ����δ�ɶԵ�����2����
����δ�ɶԵ��Ӹ���������Cr����ѡd��
��2�����йµ��Ӻͺ��пչ����ԭ��֮�������λ������λ���ɺ��йµ��ӶԵ�ԭ��ָ���пչ����ԭ�ӣ������������λ��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��C��HԪ���γɵ�ij�л�����������й���16�����ӣ�ÿ��̼ԭ�Ӻ���6�����ӣ����Ըû���������ຬ������̼ԭ�ӣ�����4��Hԭ�ӣ�Ϊ��ϩ�����۵���Ϊ�Ҽ�������˫����һ���ǦҼ�һ���Ǧм����÷����ЦҼ�5�����м�1�������Զ���֮��Ϊ5��1��
���л���������һ���������γɵľۺ�����Cԭ�Ӽ۲���ӶԸ�����4�����ݼ۲���ӶԻ�������֪̼ԭ���ӻ���ʽΪsp3��
�ʴ�Ϊ��5��1��sp3��
��4���þ�����Nԭ�Ӹ���=8��$\frac{1}{8}$=1��Cuԭ�Ӹ���=12��$\frac{1}{4}$=3�������仯ѧʽΪCu3N�������ı߳�Ϊa pm�������=a3pm3���ܶ�=$\frac{\frac{M}{{N}_{A}}��1}{V}$=$\frac{\frac{206}{{N}_{A}}��1}{{a}^{3}}$g��pm-3=$\frac{206}{{a}^{3}{N}_{A}}$g��pm-3��
�ʴ�Ϊ��$\frac{206}{{a}^{3}{N}_{A}}$��
���� ���⿼�����ʽṹ�����ʣ����ؿ���ѧ�����㡢�ռ������������漰�������㡢ԭ���ӻ���ʽ�жϡ���λ����ԭ�Ӻ�������Ų���֪ʶ�㣬��Щ���Ǹ�Ƶ���㣬�ѵ��ǣ�4�����ܶȼ��㣬��Ŀ�Ѷ��еȣ�

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д� ��������ľ��ԭ�ϣ�����Χ�ƴ����ʵ�������ȷ���ǣ�������
��������ľ��ԭ�ϣ�����Χ�ƴ����ʵ�������ȷ���ǣ�������| A�� | ���л���û��ȷ�����۵� | B�� | ���л���ͨ���Ӿ۷�Ӧ�õ� | ||
| C�� | ���л���ͨ�����Ӻͼ״���Ӧ�õ� | D�� | ���л���ĵ�����-C6H3OHCH2- |
| A�� | ��l0mL0.1mol•L-1FeI����Һ�л���ͨ�˱�״����11.2mL Cl2 | |
| B�� | ��AlCl3��Һ�еμ�Na2S��Һ | |
| C�� | ����Na+��Fe2+��Cl-��SO42-���ӵ���Һ��ͨ��NO2 | |
| D�� | ��5mL0.1 mol•L-1NaOH��Һ�У���μ���1 mL0.1 mol•L-1AlCl3��Һ���ߵμӱ��� |
 �����ͬ���ܱ������о�����1mol X��1mol Y���ֱ���300���500�濪ʼ������Ӧ��X��g��+Y��g��?3Z��g����Z�ĺ�����Z%����ʱ��t�ı仯��ͼ��ʾ����֪��t3ʱ�̸ı�����bijһʵ�������������ж���ȷ���ǣ�������
�����ͬ���ܱ������о�����1mol X��1mol Y���ֱ���300���500�濪ʼ������Ӧ��X��g��+Y��g��?3Z��g����Z�ĺ�����Z%����ʱ��t�ı仯��ͼ��ʾ����֪��t3ʱ�̸ı�����bijһʵ�������������ж���ȷ���ǣ�������| A�� | ����a��500��ʱ��ͼ�� | |
| B�� | ��0��t1ʱ�̣���Ӧ��X��g����$\frac{v��300�棩}{v��500�棩}$��1 | |
| C�� | t2ʱ��������Z����$\frac{{��v}_{1}��300�棩}{{��v}_{1}��500�棩}$ | |
| D�� | t3ʱ�̸ı�����������ǽ��� |
| A�� | ֻ��֤����ȫȼ�պ�IJ���ֻ��H2O��CO2 | |
| B�� | ֻ�ⶨ��ȼ�ղ�����H2O��CO2�����ʵ����ı�ֵ | |
| C�� | �ⶨ��ȫȼ��ʱ���ĵ��л��������ɵ�CO2��H2O�����ʵ���֮�� | |
| D�� | �ⶨ����������������������ȫȼ�պ�ֻ����CO2��H2O���ⶨCO2��H2O������ |
| A�� | Li��Na��KԪ�ص�ԭ�Ӻ�����Ӳ������ź˵���������Ӷ����� | |
| B�� | �ڶ�����Ԫ�ش�Li��F���ǽ���������ǿ | |
| C�� | ��ΪAl��Mgʧȥ�������࣬����Al��Mg�Ļ�ԭ��ǿ | |
| D�� | O��SΪͬ����Ԫ�أ���O��S�ķǽ�����ǿ |
 ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺
ij�о���ѧϰС��Ϊ̽��п�����ᷴӦ��ȡͬ������ͬ�����пƬ��ͬŨ��������������ƽ��ʵ�飺
 ��
�� ������һ�֣���
������һ�֣��� ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +nH2O��
+nH2O�� ��
��