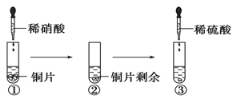
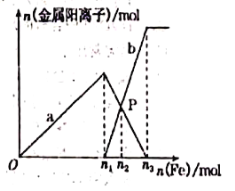
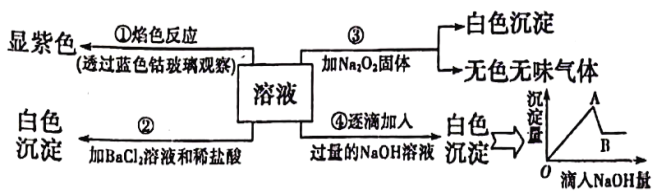
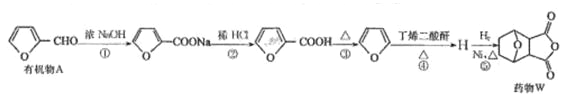
��Ŀ����
����Ŀ���������ֳƼ��ѣ����DME���۵�Ϊ-141.5�棬�е�Ϊ-24.9�档��������Һ��ʯ����(LPG)���ƣ�����Ϊ��21���͵����ȼ�ϡ����ɺϳ���(CO��H2)�Ʊ������ѵķ�Ӧԭ�����£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1
CH3OH(g) ��H1= -90.0 kJ��mol-1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
�ش��������⣺
��1����Ӧ��Ϊ��___________(����ӡ����١�)�ķ�Ӧ��__________ (����¡����¡�)�����Է����С�
��2��д���ɺϳ���(CO��H2) ֱ���Ʊ������ѵ��Ȼ�ѧ����ʽ��__________________________________��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ�١��ڣ�5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1����H2��ʾ��Ӧ�ٵ�������______________����Ӧ�ڵ�ƽ�ⳣ��K=________________��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱ��Ӧ�ڵ�v��__ v��(����>���� ��< ������=��)��
��4���о����֣��������ͬ�������м������ʵ�����ͬ��CO��H2������Ӧ�١��ڣ��ڲ�ͬ�¶Ⱥ�����������¾�����ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | COת����(%) | CH3OCH3ѡ����(%) |
473 | �� | 10 | 36 |
500 | �� | 12 | 39 |
500 | Cu/ZnO | 20 | 81 |
[��ע]������ѡ���ԣ�ת����CO������CH3OCH3�İٷֱ�
����ͬ�¶��£�ѡ��Cu/ZnO���������ô�����____________ (����)��
A.�ٽ�ƽ�������ƶ� B.��߷�Ӧ���� C.���ͷ�Ӧ�Ļ��
D.�ı䷴Ӧ���ʱ� E.���CO��ƽ��ת����
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬��ԭ����_________________________________________________��
���𰸡� ��С ���� 2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l 0.24mol/(L��min) 1 < B��C �ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3
CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l 0.24mol/(L��min) 1 < B��C �ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3
����������1����Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1 ������������٣����ؼ��٣�����S<0���÷�ӦΪ���ȷ�Ӧ��Ҫ���Է����У���H-T��S<0��T������ѧ�¶ȣ�����ֵ���ʵ���ʱ����H-T��S<0���÷�Ӧ�ڵ����������Է����У�
CH3OH(g) ��H1= -90.0 kJ��mol-1 ������������٣����ؼ��٣�����S<0���÷�ӦΪ���ȷ�Ӧ��Ҫ���Է����У���H-T��S<0��T������ѧ�¶ȣ�����ֵ���ʵ���ʱ����H-T��S<0���÷�Ӧ�ڵ����������Է����У�
��2���ɺϳ���(CO��H2) ֱ���Ʊ������ѵĻ�ѧ����ʽ��2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g)���÷�Ӧ���ɢ١�2+�ڵ������ʸ÷�Ӧ�ġ�H=-200 kJ��mol-l���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��2CO(g)+4H2(g)
CH3OCH3(g)+H2O(g)���÷�Ӧ���ɢ١�2+�ڵ������ʸ÷�Ӧ�ġ�H=-200 kJ��mol-l���ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ��2CO(g)+4H2(g)![]() CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l��
CH3OCH3(g)+H2O(g) ��H= -200 kJ��mol-l��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ��������5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1��
��CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼŨ�� 1 3 0
��ӦŨ�� 0.6 1.2 0.6
ƽ��Ũ�� 0.4 1.8 0.6
H2��ʾ��Ӧ�ٵ�������v=![]() =0.24mol/(L��min)
=0.24mol/(L��min)
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g)
CH3OCH3(g)+H2O(g)
��ʼŨ�� 0.6 0 0
��ӦŨ�� 0.4 0.2 0.2
ƽ��Ũ�� 0.2 0.2 0.2
��Ӧ�ڵ�ƽ�ⳣ��K= ![]() = 1��
= 1��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱn(CH3OCH3)=n��H2O��=2n (CH3OH)��c(CH3OCH3)=c��H2O��=2c (CH3OH)����ʱQc=0.25>K��ƽ�⽫�����ƶ�����ʱ
��Ӧ�ڵ�v��< v����
��4�����ݱ������ͬ�¶��£�ѡ��Cu/ZnO������������ֻ�ܼӿ췴Ӧ���ʡ����ͷ�Ӧ��ܣ���ѡB��C��
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬�ɱ������ݿ�֪����ʱ��Ӧ��ϵ��δ����ƽ�⣬�����ɼ��ٷ�Ӧ�ڣ��Ӷ�����ͬʱ�������ɸ����CH3OCH3��

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�