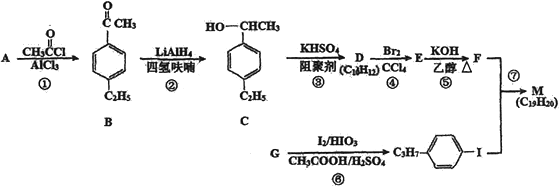
��Ŀ����
����Ŀ����ҵ�Ϸ�������Ϊ���ȼ���е���������1886�귨����ѧ��H��M0issanͨ�������⻯��(KHF2)�ķ�������ˮ��Һ��һ���Ƶ÷�������֪��KF+HF=KHF2���Ʊ������ĵ��װ����ͼ��ʾ������˵���������
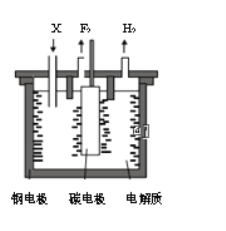
A. �ֵ缫���Դ�ĸ�������
B. �������費�ϲ����X��KF
C. �������������ұ������
D. ���⻯���ڷ������п��Ե���
���𰸡�B
��������
������ڵķ��⻯�أ�KHF2���ͷ����⣨HF��������Ʊ������ʣ�������������ʧ�������ɷ�����������KHF2�õ����������������缫��ӦΪ��2HF2-+2e-�TH2��+4F-��A. ����ͼʾ���ֵ缫�ϲ���������������ԭ��Ӧ���ֵ缫Ϊ���������Դ�ĸ�����������A��ȷ��B. �������⣬�������л�����ķ��⻯�أ�KHF2�����費�ϲ����X�Ƿ��⻯�أ�KHF2������B����C. ����Ʒ�ʱ��Ϊ�˷�ֹ�����������������ҷ�Ӧ��������ը���������������ұ����������C��ȷ��D. �����������������⻯���ڷ������п��Ե����HF2-����D��ȷ����ѡB��

 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�����Ŀ���������ֳƼ��ѣ����DME���۵�Ϊ-141.5�棬�е�Ϊ-24.9�档��������Һ��ʯ����(LPG)���ƣ�����Ϊ��21���͵����ȼ�ϡ����ɺϳ���(CO��H2)�Ʊ������ѵķ�Ӧԭ�����£�
��CO(g)+2H2(g)![]() CH3OH(g) ��H1= -90.0 kJ��mol-1
CH3OH(g) ��H1= -90.0 kJ��mol-1
��2CH3OH(g)![]() CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
CH3OCH3(g)+H2O(g) ��H2=-20.0kJ��mol-l
�ش��������⣺
��1����Ӧ��Ϊ��___________(����ӡ����١�)�ķ�Ӧ��__________ (����¡����¡�)�����Է����С�
��2��д���ɺϳ���(CO��H2) ֱ���Ʊ������ѵ��Ȼ�ѧ����ʽ��__________________________________��
��3���¶�Ϊ500Kʱ����2L���ܱ������г���2 mol CO��6 molH2������Ӧ�١��ڣ�5 minʱ�ﵽƽ�⣬ƽ��ʱCO��ת����Ϊ60%��c(CH3OCH3)=0.2 mol��L-1����H2��ʾ��Ӧ�ٵ�������______________����Ӧ�ڵ�ƽ�ⳣ��K=________________��
����500Kʱ�����������n(CH3OCH3)=2n (CH3OH)����ʱ��Ӧ�ڵ�v��__ v��(����>���� ��< ������=��)��
��4���о����֣��������ͬ�������м������ʵ�����ͬ��CO��H2������Ӧ�١��ڣ��ڲ�ͬ�¶Ⱥ�����������¾�����ͬ��Ӧʱ��������ʵ�����ݣ�
T(K) | ���� | COת����(%) | CH3OCH3ѡ����(%) |
473 | �� | 10 | 36 |
500 | �� | 12 | 39 |
500 | Cu/ZnO | 20 | 81 |
[��ע]������ѡ���ԣ�ת����CO������CH3OCH3�İٷֱ�
����ͬ�¶��£�ѡ��Cu/ZnO���������ô�����____________ (����)��
A.�ٽ�ƽ�������ƶ� B.��߷�Ӧ���� C.���ͷ�Ӧ�Ļ��
D.�ı䷴Ӧ���ʱ� E.���CO��ƽ��ת����
�ڱ���ʵ�����ݱ�������500Kʱ������Cu/ZnO��COת����CH3OCH3��ѡ������������Ӱ�죬��ԭ����_________________________________________________��
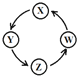
����Ŀ�����и��������У��������ͼʾ������һ����������һ��ת���������( )
��� | X | Y | Z | W |
|
�� | Cu | CuSO4 | Cu(OH)2 | CuO | |
�� | Na | NaOH | Na2CO3 | NaCl | |
�� | Al | AlCl3 | Al(OH)3 | Al2O3 | |
�� | Fe | FeCl3 | FeCl2 | Fe(OH)2 |
A. �ڢۢ�B. �٢ۢ�C. �٢ڢ�D. �٢ڢ�