��Ŀ����
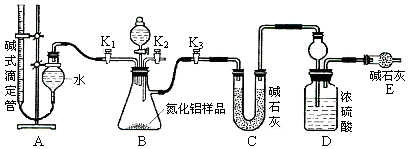
CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��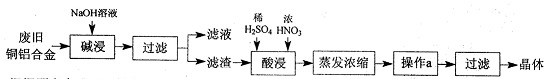
�����������������գ�
��1�����������Ŀ���� ��д���йص����ӷ���ʽ ��
��2�����������ȼ�������ϡ���ᣬȻ���ٵμ�����Ũ���ᣬ�ڷ����ܽ�ʱ���Թ۲쵽��ʵ�������� ��
��3������a������Ϊ ���Ƶõ�CuSO4��5H2O�п��ܴ�������ͭ���ʣ���ȥ�������ʵ�ʵ���������Ϊ ��
��4����֪��CuSO4+2NaOH=Cu��OH��2��+ Na2SO4����ȡ0��26 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0��1000 mol/L����������Һ28��00 mL����Ӧ��ȫ����������������0��1000 mol/L����ζ����յ㣬��������8��00 mL�����������CuSO4��5H2O����������Ϊ �������ζ��У��ζ�����ע������֮ǰ����������ˮϴ�������� ��
��5���ڡ�������IJ����У�����ֻ����Ũ���ᣬд������ʱ�Ļ�ѧ����ʽ ��
������Ũ���ỻ�ɹ������⣬����ʱ������������ͭ��ָ�����ַ������ŵ� ��
��1���ܽ����������ȥ���� 2Al+2H2O+2OH-=2AlO2-+3H2��
��2�������ܽ⣬��Һ����������ɫ�������������Һ�Ϸ���Ϊ����ɫ
��3����ȴ�ᾧ �ؽᾧ
��4��0.92 �ñ�������ϴ2~3��
��5��Cu+2H2SO4(Ũ)==CuSO4+SO2��+2H2O
���������������1��ʵ��Ŀ�������÷Ͼ���ͭ�Ͻ��Ʊ�����ͭ���壬��Ӧ�ȳ��ӡ�������dz��ӹ��̣���ȥ��������ۺ��ܽ�����������ӷ���ʽΪ2Al+2H2O+2OH-=2AlO2-+3H2������2����������Ũ�����ϡ�ͣ���ͭ��Ӧ����������ͭ��NO���壬������Ϊͭ�ܽ⣬��Һ��Ϊ��ɫ������ɫ���������ֻ��Һ�Ϸ���Ϊ����ɫ�����NO2������3��������ȴ�ᾧ�ķ�������������ͭ���壬��ȥ�����еĿ��������ʵķ������ؽᾧ��
��4����CuSO4���������Ƶ���n(NaOH)=28.00��0.1000��10-3��8.00��0.1000��10-3=2��10-3mol
n(CuSO4��5H2O) = n(NaOH)/2=10-3mol w(CuSO4��5H2O)=10-3��250��0.26=0.92
(5)����������һ����ɫ�����������Ũ������Բ�������Ⱦ�����壬����������
���㣺���鹤ҵ�������йز���Ŀ�ġ�����������ʵ��������й����⡣

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д���ѧ��һ����ʵ��Ϊ�����Ŀ�ѧ��
��1��������������ȷ����______������ţ���
| A����Һ©�����ζ��ܺ�����ƿʹ��ǰ�������Ƿ�©ˮ |
| B������ˮ�����Һ©�����ټ������Ҵ�����������ã��ɴӵ�ˮ����ȡ�� |
| C���ྻ��������ʳ��ˮ�н���һ��ʱ�䣬�����������ݣ�˵�������������ⸯʴ |
| D����˿�������о���ȼ�գ��������䣬���ɺ�ɫ���� |
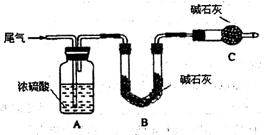
��2����ͭƬ��ϡ���ᷴӦ��ȡNO���壬��ͼװ�����ʺϵ���______������ţ���װ��B�е��Լ������______����װ�õ�������______________________��

���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȡ�����д���пհף�
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȡ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��
��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������±���
| | �������� | ����/g |
| ��ƿ��ˮ������ | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |
�ټ���CaC2����������ʱ����������6�ζ�����ԭ���ǣ� ��
�ڴ�������CaC2����������Ϊ ��(����2λ��Ч����)
��3�������ַ�������ȡһ��������������1.60g���������������£�

�ٲ������������ ��
����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ��� ���ƫ����ƫС�����䡱����
���������к���CO��CO2��SO2��H2O�����壬�â���ˮCuSO4���ڳ���ʯ��ˮ�ۺ���CuO������ʯ�ҡ���Ʒ����Һ�������Ը��������Һ��ҩƷ�ɽ���һһ��������ʱ��������ͨ��ҩƷ����ȷ˳����(����)
| A���٢ݢڢ٢ܢ� | B���٢ݢޢڢܢ� |
| C���ۢܢڢޢݢ� | D���ڢݢ٢ܢۢ� |
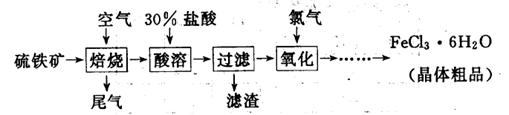
 6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .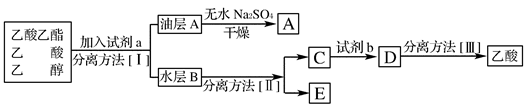



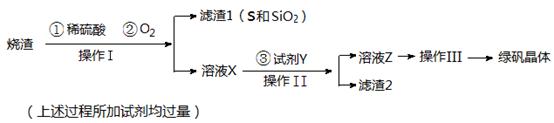 �Իش�
�Իش�