��Ŀ����
����Ŀ����ͼ�У�AΪһ�ֳ����ĵ��ʣ�B��C��D��E�Ǻ���AԪ�صij�����������ǵ���ɫʵ���Ϊ��ɫ��
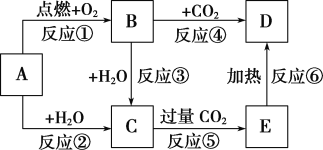
(1)д���������ʵĻ�ѧʽ��B________��E___________��
(2)����6����Ӧ������������ԭ��Ӧ����________(��д���)��
(3)д��B��C��Ӧ�����ӷ���ʽ��________________________________________��
C��E�Ļ�ѧ����ʽ��____________________________________________��
E��D�Ļ�ѧ����ʽ��___________________________________________��
(4)����5.00 g D��E�Ĺ������ʹE��ȫ�ֽ⣬�������������������0.31 g����ԭ�������D������Ϊ_________��
���𰸡�Na2O2 NaHCO3 �٢ڢۢ� 2Na2O2��2H2O��4Na����4OH����O2�� NaOH��CO2��NaHCO3 2NaHCO3![]() Na2CO3��CO2����H2O 4.16 g
Na2CO3��CO2����H2O 4.16 g
��������
AΪһ�ֳ����ĵ��ʣ�B��C��D��E�Ǻ���AԪ�صij�����������ǵ���ɫ��Ӧ��Ϊ��ɫ�����Ϊ�Ƶĵ��ʻ������AΪNa�����ת����ϵ��֪��BΪNa2O2��CΪNaOH��DΪNa2CO3��EΪNaHCO3��
AΪNa�����ת����ϵ��֪��BΪNa2O2��CΪNaOH��DΪNa2CO3��EΪNaHCO3��
��1����������ķ�����֪��BΪNa2O2��EΪNaHCO3���ʴ�Ϊ��Na2O2��NaHCO3��
��2�����Ϸ�Ӧ�Т�Ϊ�Ƶ�ȼ�գ���ΪNa��ˮ��Ӧ����Ϊ����������ˮ��Ӧ����Ϊ���������������̼��Ӧ��������������ԭ��Ӧ���ʴ�Ϊ���٢ڢۢܣ�
��3��B��C�Ļ�ѧ����ʽΪ2Na2O2+2H2O��4NaOH+O2�������ӷ���ʽΪ2Na2O2��2H2O��4Na����4OH����O2����C��E��Ӧ�Ļ�ѧ����ʽΪ��NaOH��CO2��NaHCO3��E��D�Ļ�ѧ����ʽ��2NaHCO3![]() Na2CO3��CO2����H2O��
Na2CO3��CO2����H2O��
��4��̼���ƽ��ȶ������Ȳ��ֽ⣬ֻ��̼�����Ʒֽ⣬�������к���xg NaHCO3����
2NaHCO3 ![]() Na2CO3+CO2��+H2O ��m
Na2CO3+CO2��+H2O ��m
2��84 62
xg 0.31g
2��84��62=xg��0.31g�����x=0.84������m��Na2CO3��=5g-m��NaHCO3��=5g-0.84g=4.16g��

����Ŀ���±���A��B��C��D�����л�����й���Ϣ��
A | ����ʹ������Ȼ�̼��Һ��ɫ�� ������ˮ��һ�������·�Ӧ����C�� �۱���ģ��Ϊ�� |
B | ����C��H����Ԫ����ɣ� �����ģ��Ϊ�� |
C | ����C��H��O����Ԫ����ɣ� ������Na��Ӧ����������NaOH��Һ��Ӧ�� ��������ѻ�Ϊͬ���칹�塣 |
D | ����Է���������C������2�� ����������C�������ɡ� |
���ݱ�����Ϣ�ش��������⣺
��1��A��������Ȼ�̼��Һ��Ӧ�������������Ϊ___________��ϵͳ����������д������������������NaOHˮ��Һ���ȵĻ�ѧ����ʽ___________��
��2��A�����������ӳɷ�Ӧ�����ɷ���F����F�ڷ�����ɺͽṹ�����Ƶ��л�����һ����(�׳���ͬϵ����)�����Ǿ�����ͨʽCnH2n��2����n��________ʱ�������л��↑ʼ����ͬ���칹�塣
��3��B���е�������________(�����)������ɫ��ζҺ�塢���ж����۲�����ˮ�����ܶȱ�ˮ�ݸ������������Һ����ˮ��Ӧ��ɫ�����κ������²���������Ӧ��д����Ũ���������£�B��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ: _______
��4��д����C��������D�Ļ�ѧ����ʽ________��
����Ŀ����������ʵ������������ܵó���Ӧ���۵���
ѡ�� | ʵ����� | ���� | ���� |
A |
| ��Һ��ɫ��ȥ |
|
B | ��1mL 2mol/L | �����ɰ�ɫ�����������ɺ��ɫ���� |
|
C | �� | ��Һ��Ϊ��ɫ | ��Ʒ�Ѳ��ֻ�ȫ������ |
D | ��ˮ�Ҵ��м���Ũ���ᣬ���ȣ�����������Yͨ������ | ��Һ��ɫ��ȥ | ����Y�к�����ϩ |
A. AB. BC. CD. D



