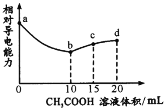
��Ŀ����
����Ŀ���л���K��һ������ȱѪ������ҩ���ϳ�·�����£�
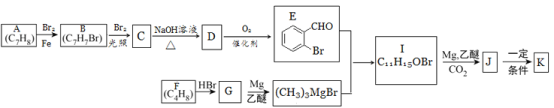
��֪����.�л���K��һ�����������г����������1����Ԫ����
��.R��Br ![]() RMgBr
RMgBr 
�ش��������⣺
(1)�л���B������Ϊ________��D�ĺ��������ŵ�������______��
(2)F��G�ķ�Ӧ����Ϊ_____��
(3)J�Ľṹ��ʽΪ______��K�ķ���ʽΪ_____��
(4)E�������Һ��Ӧ�Ļ�ѧ����ʽΪ______��
(5)��֪B��һ�������¿���ˮ������X����X��Ϊͬ���칹���Һ��б������л�����_____��(����X����)��д�����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽ____(��дһ��)��
(6)����������Ϣ��ѧ֪ʶ��д���Լ���ͱ���ȩΪԭ�ϣ��ϳɱ���ϩ��·������ͼ(�����Լ���ѡ)____��
���𰸡�����ױ���2-��ױ� �ǻ� �ӳɷ�Ӧ ![]() C12H14O2
C12H14O2 ![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O 4
+2Ag��+3NH3+H2O 4 ![]() ��
��![]() CH4
CH4![]() CH3Br
CH3Br![]() CH3MgBr��
CH3MgBr�� ![]()
![]()
![]()
![]()
![]()
��������
��A-E�Ŀ�ͼ���ƿ�֪��DΪ![]() ��CΪ
��CΪ![]() ��BΪ
��BΪ![]() ����F-G�ͷ�Ӧ������֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br����������֪�ͷ�Ӧ����֪I�Ľṹ��ʽΪ
����F-G�ͷ�Ӧ������֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br����������֪�ͷ�Ӧ����֪I�Ľṹ��ʽΪ![]() ��J�Ľṹ��ʽΪ
��J�Ľṹ��ʽΪ![]() ��K�Ľṹ��ʽΪ
��K�Ľṹ��ʽΪ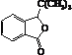 ��
��
��1�����л���B�Ľṹ��ʽ![]() ����֪B������Ϊ����ױ���D�Ľṹ��ʽ��
����֪B������Ϊ����ױ���D�Ľṹ��ʽ��![]() �����������ŵ��������ǻ����ʱ����Ϊ������ױ���2-��ױ����ǻ���
�����������ŵ��������ǻ����ʱ����Ϊ������ױ���2-��ױ����ǻ���
��2�����ݷ�Ӧ������F��G�Ľṹ��֪��FΪCH2=C(CH3)2��HBr�ӳ�����G��CH3C(CH3)2Br��������F��G�ķ�Ӧ����Ϊ�ӳɷ�Ӧ���ʱ����Ϊ���ӳɷ�Ӧ��
��3�����ݷ���֪/span>J�Ľṹ��ʽΪ![]() ��K�ķ���ʽΪC12H14O2���ʱ����Ϊ��
��K�ķ���ʽΪC12H14O2���ʱ����Ϊ��![]() ��C12H14O2��
��C12H14O2��
��4���ɷ���֪EΪ![]() ����������Һ��Ӧ�Ļ�ѧ����ʽΪ��
����������Һ��Ӧ�Ļ�ѧ����ʽΪ��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O���ʱ����Ϊ��
+2Ag��+3NH3+H2O���ʱ����Ϊ��![]() +2Ag(NH3)2OH
+2Ag(NH3)2OH ![]()
![]() +2Ag��+3NH3+H2O��
+2Ag��+3NH3+H2O��
��5���л���B(�ṹ��ʽΪ![]() )��һ��������ˮ��IJ���XΪ
)��һ��������ˮ��IJ���XΪ![]() �����以Ϊͬ���칹���Һ��б������л����У�
�����以Ϊͬ���칹���Һ��б������л����У�![]() ��
��![]() ��
��![]() ��
��![]() ����4�֣����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽΪ
����4�֣����к˴Ź���������4�����շ壬�ҷ�ֵ��Ϊ3��2��2��1�Ľṹ��ʽΪ![]() ��
��![]() ���ʱ����Ϊ��4��
���ʱ����Ϊ��4��![]() ��
��![]() ��
��
��6���Լ���ͻ�����A��![]() ��Ϊԭ�ϣ��ϳɱ���ϩ��·������ͼ��CH4
��Ϊԭ�ϣ��ϳɱ���ϩ��·������ͼ��CH4![]() CH3Br
CH3Br![]() CH3MgBr���ɱ���ȩ��
CH3MgBr���ɱ���ȩ��![]() ��Ϊԭ�ϣ�
��Ϊԭ�ϣ�![]()
![]()
![]()
![]()
![]() ���ʱ����Ϊ��CH4
���ʱ����Ϊ��CH4![]() CH3Br
CH3Br![]() CH3MgBr��
CH3MgBr�� ![]()
![]()
![]()
![]()
![]() ��
��

����Ŀ��ij��ʯ����Ҫ�ɷ���BaCO3(��Ca2+��Mg2+��Fe3+������)��ʵ�������øÿ�ʯ�Ʊ�BaCl2��2H2O��������ͼ��
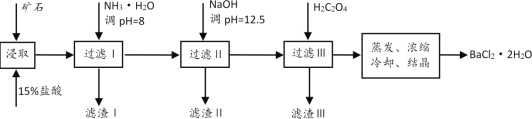
(1)��ϡ�����ȡǰ������ĥ��ʯ��Ŀ����__��
(2)����NH3��H2O����pH=8�ɳ�ȥ__(�����ӷ���)���������к�__(�ѧʽ)��
Ca2+ | Mg2+ | Fe3+ | |
��ʼ����ʱ��pH | 11.9 | 9.1 | 1.9 |
��ȫ����ʱ��pH | 13.9 | 11.1 | 3.2 |
(3)ҪʹCa2+��ȫ������Ӧ������Һ�е�![]() ��Ũ�Ȳ�����__mol/L(����Ũ��С��1��10-5mol/Lʱ����Ϊ�����ʹ���ȫ)��ͬʱ����H2C2O4ʱ��Ӧ���������ԭ����__��(��֪��KSP(BaC2O4=1.6��10-7��KSP(CaC2O4=2.3��10-9)��
��Ũ�Ȳ�����__mol/L(����Ũ��С��1��10-5mol/Lʱ����Ϊ�����ʹ���ȫ)��ͬʱ����H2C2O4ʱ��Ӧ���������ԭ����__��(��֪��KSP(BaC2O4=1.6��10-7��KSP(CaC2O4=2.3��10-9)��
(4)����������ԭ�ζ����ɲⶨH2C2O4��Ũ�ȣ�ȡ20.00mLH2C2O4��Һ����ƿ�У���0.10mol/L����KMnO4��Һ�ζ���KMnO4��ҺӦװ��__(������ʽ�ζ�����������ʽ�ζ�����)�С�д����Ӧ�����ӷ���ʽΪ__���ζ��յ������Ϊ__�����ζ��ﵽ�յ�ʱ������KMnO4��Һ30.00mL����H2C2O4��Һ��Ũ��Ϊ__mol/L��