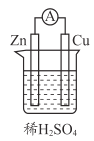
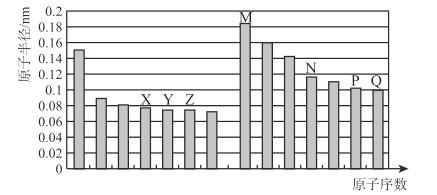
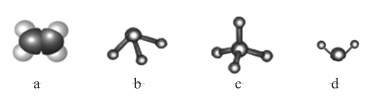
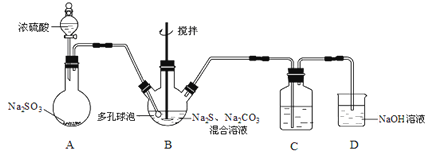
��Ŀ����
����Ŀ����֪������������ճ������м�Ϊ�������ᣬ��һ�������£�CH3COOH��Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO+H+ ��H��0��
CH3COO+H+ ��H��0��
��1�����³�ѹ�£���pH=5��ϡ������Һ�У�c(CH3COO��=__������ȷֵ�����з����У�����ʹ0.10mol��L��1CH3COOH�ĵ���̶��������___��
a.��������0.10mol��L1��ϡ���� b.����CH3COOH��Һ c.�������������� d.��ˮϡ����0.010mol��L��1 e.���������Ȼ��ƹ��� f.��������0.10mol��L��1��NaOH��Һ
��2����֪��90��ʱ��ˮ�����ӻ�����ΪKw=38��10��14���ڴ��¶��£���pH=3�������pH=11������������Һ�������ϣ�������Һ�е�c(H+)=___��������λ��Ч���֣���
��3������Ũ�Ⱦ�Ϊ0.1mol/L��������Һ�������� �ڴ��� ���������� ���Ȼ�� �ݴ���� ������� ��������� �ఱˮ����ش��������⣺
��.�١��ڡ��ۡ���������Һ����ˮ�������H����Ũ���ɴ�С��˳����(�����)__��
��.�ܡ��ݡ��ޡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳����(�����)___��
��4��һ�������¼״���һ����̼��Ӧ���Ժϳ����ᡣ���������£���a mol/L��CH3COOH��b mol/LBa��OH��2��Һ�������ϣ���Ӧƽ��ʱ��2c��Ba2+��=c��CH3COO-�����ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ___��
���𰸡���10��5��10��9��mol��L��1 bdf 2.05��10��11mol��L-1 �ܢڢۢ� �ޢߢܢݢ� ![]()
��������
��1����Һ��H�����������������Ļ���ˮ��������ġ�����ˮ�����ӻ���֪��ˮ���������c��H����=c��OH����=![]() =10��9mol��L��1����˴�����������c��H����=c��CH3COO����=��10��5��10��9��mol��L��1��������ڵĵ���ƽ��ΪCH3COOH
=10��9mol��L��1����˴�����������c��H����=c��CH3COO����=��10��5��10��9��mol��L��1��������ڵĵ���ƽ��ΪCH3COOH![]() CH3COO����H������
CH3COO����H������
a������������������Һc(H��)���Դ���ĵ������������ã�a����
b��������ʵĵ��������ȷ�Ӧ�������¶ȣ�����������뷽����У�b��ȷ��
c����������ᣬ����̶Ƚ��ͣ�c����
d����ˮϡ�ͣ�������Ũ�ȣ�������������ԭ����ƽ����Ũ������ķ�����У�������뷽����У�d��ȷ��
e�������Ȼ��ƹ��壬�Ե�����Ӱ�죬e����
f�������������ƣ�����H������ʹƽ������뷽����У�f��ȷ���ʴ�ѡbdf��
��2��pH=3��������c��H+��=10-3mol/L��pH=11������������Һ��c��OH-��=![]() =38��10-3mol/L����Ϻ�H++OH-=H2O�����Լ�ʣ�࣬ʣ�����������Ũ��Ϊc��OH-��=
=38��10-3mol/L����Ϻ�H++OH-=H2O�����Լ�ʣ�࣬ʣ�����������Ũ��Ϊc��OH-��=![]() =0.0185mol/L������c��H+��=
=0.0185mol/L������c��H+��=![]() ��2.05��10-11 mol��L-1��
��2.05��10-11 mol��L-1��
��3����.����ʹ��ᡢ�������ƾ�����ˮ�ĵ��룬��Ũ�ȵ�����ʹ��ᡢ������������Һ�е������H+��OH-Ũ�ȴ�С��ϵ��H2SO4��NaOH��CH3COOH����˶�ˮ�ĵ�����������ǿ����ϵΪ��H2SO4��NaOH��CH3COOH����ˮ���������H+Ũ�ȴ�С��ϵ��CH3COOH��NaOH��H2SO4������ΪNH4Cl�ܹ�ˮ�⣬��ˮ�ĵ�����ٽ����ã�����������Һ����ˮ�������H+Ũ���ɴ�С��˳��NH4Cl��CH3COOH��NaOH��H2SO4�����ܢڢۢ١�
��.�Ȼ�狀��������Һ�д�����NH4Cl=NH4++Cl-��NH4++H2O![]() NH3
NH3![]() H2O+H+���������Һ��CH3COONH4=CH3COO-+NH4+��CH3COO-+H2O
H2O+H+���������Һ��CH3COONH4=CH3COO-+NH4+��CH3COO-+H2O![]() CH3COOH+OH-��NH4++H2O
CH3COOH+OH-��NH4++H2O![]() NH3
NH3![]() H2O+H+�����������Һ��NH4HSO4=NH4++H++SO42-��NH4++H2O
H2O+H+�����������Һ��NH4HSO4=NH4++H++SO42-��NH4++H2O![]() NH3
NH3![]() H2O+H+����ˮ��NH3
H2O+H+����ˮ��NH3![]() H2O
H2O![]() NH4++OH-����Ũ�ȵ��������ʣ��������NH4+�ij�ʼŨ������Ȼ����Һ���������Һ�����������Һ��NH4+�ij�ʼŨ����ͬ���������CH3COO-ˮ�����ɵ�OH-��NH4+��ˮ����ٽ����ã����������H+��NH4+��ˮ�����������ã�������֮��NH4+Ũ�ȴ�СΪ��NH4HSO4��NH4Cl��CH3COONH4������Ϊ��ˮֻ�ܲ��ֵ��룬����������Һ�к��е�NH4+Ũ����С����������������Һ��NH4+Ũ���ɴ�С��˳���ǣ��ޢߢܢݢࣻ
NH4++OH-����Ũ�ȵ��������ʣ��������NH4+�ij�ʼŨ������Ȼ����Һ���������Һ�����������Һ��NH4+�ij�ʼŨ����ͬ���������CH3COO-ˮ�����ɵ�OH-��NH4+��ˮ����ٽ����ã����������H+��NH4+��ˮ�����������ã�������֮��NH4+Ũ�ȴ�СΪ��NH4HSO4��NH4Cl��CH3COONH4������Ϊ��ˮֻ�ܲ��ֵ��룬����������Һ�к��е�NH4+Ũ����С����������������Һ��NH4+Ũ���ɴ�С��˳���ǣ��ޢߢܢݢࣻ
��4�����������£���a mol/L��CH3COOH��b mol/L Ba(OH)2��Һ�������ϣ�������Һ���ֵ����ԣ�2c(Ba2��)+c(H+)��c(CH3COO��)+c(OH��)����2c(Ba2��)��c(CH3COO��)��b mol/L������Һ�������ԣ���c(H+)=c(OH��)=1��10-7mol/L�����ݴ���������������Һ��Ӧ�Ļ�ѧ����ʽȷ�����Һ��c(CH3COOH)=![]() mol/L���û����Һ�д���ĵ��볣��Ϊ
mol/L���û����Һ�д���ĵ��볣��Ϊ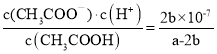 ��
��

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�