��Ŀ����
����Ŀ��X��Y��Z��W������ԭ��������������Ķ�����Ԫ�أ�W������������ΪX������������һ�룬X��Y��Z��ԭ�Ӱ뾶���μ�С��X��Y��Z��ɵ�һ�ֻ�����(ZXY)2�ĽṹʽΪY��X��Z��Z��X��Y������˵����ȷ����
A.(XY)2��XԪ�صĻ��ϼ�Ϊ+3
B.Y���������Ӧ��ˮ������ǿ��
C.������W(Y3)2��ֻ�������Ӽ�
D.X��Z��ɵĻ������в���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ
���𰸡�A
��������
X��Y��Z��W������ԭ��������������Ķ�����Ԫ�أ�X��Y��Z��ԭ�Ӱ뾶���μ�С��X��Y��Z��ɵ�һ�ֻ�����(ZXY)2�ĽṹʽΪY��X��Z��Z��X��Y��˵��XΪCԪ�ء�YΪNԪ�ء�ZΪOԪ�أ�W������������ΪX������������һ�룬��WΪMgԪ�أ�
A��CԪ�صķǽ����Ա�NԪ��������(CN)2��CԪ�صĻ��ϼ�Ϊ+3����A��ȷ��
B��NԪ�ص�����������Ӧ��ˮ����HNO3��ǿ�ᣬ��+3��NԪ���������ˮ����HNO2�����ᣬ��B����
C��������Mg(N3)2�г��������Ӽ���N3-�л����зǼ��Թ��ۼ�����C����
D��C��O��ɵĻ�����CO2�ĵ���ʽΪ![]() ��CΪOԭ�Ӷ��ﵽ8�����ȶ��ṹ����D����
��CΪOԭ�Ӷ��ﵽ8�����ȶ��ṹ����D����
�ʴ�ΪA��

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�����Ŀ��H2��һ����Ҫ�������Դ��
��1����֪��CO2(g)+3H2(g)=CH3OH(g)+H2O(g) ��H2=-49.0kJ/mol��
CO(g)+H2O(g)=CO2(g)+H2(g) ��H3=-41.1kJ/mol��H2��ԭCO��Ӧ�ϳɼ״����Ȼ�ѧ����ʽΪ��CO(g)+2H2(g)![]() CH3OH(g) ��H1������H1=___kJ/mol���÷�Ӧ�Է����е�����Ϊ_____��
CH3OH(g) ��H1������H1=___kJ/mol���÷�Ӧ�Է����е�����Ϊ_____��
A.���� B.���� C.�κ��¶�������
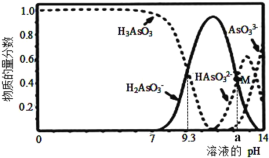
��2�����º�ѹ�£����ݻ��ɱ���ܱ������м���1molCO��2.2molH2��������ӦCO(g)+2H2(g)![]() CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
CH3OH(g)��ʵ����ƽ��ʱCO��ת�������¶ȡ�ѹǿ�ı仯��ͼ��ʾ��
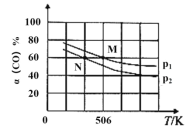
��ѹǿ��p1____p2��(����>��<������=��)
��M��ʱ��H2��ת����Ϊ_____(��������ȷ��0.1%)�� �÷�Ӧ��ƽ�ⳣ��Kp=____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ���־����______(����ĸ)��
A.������ѹǿ���ֲ��� B.2v��(H2)=v��(CH3OH)
C.����������Է����������ֲ��� D.���������ܶȱ��ֲ���
��3��H2��ԭNO�ķ�ӦΪ2NO(g)+2H2(g)![]() N2(g)+2H2O(1)��ʵ���÷�Ӧ���ʵı���ʽΪv=kcm(NO)��cn(H2)(k�����ʳ�����ֻ���¶��й�)
N2(g)+2H2O(1)��ʵ���÷�Ӧ���ʵı���ʽΪv=kcm(NO)��cn(H2)(k�����ʳ�����ֻ���¶��й�)
��ij�¶��£���Ӧ�����뷴Ӧ��Ũ�ȵı仯��ϵ���±���ʾ��
��� | c(NO)/(mol/L) | c(H2)/(mol/L) | v/(mol��L-1��min-1) |
1 | 0.10 | 0.10 | 0.414 |
2 | 0.10 | 0.20 | 1.656 |
3 | 0.50 | 0.10 | 2.070 |
�ɱ������ݿ�֪��m=_____��n=_____��
��������Ӧ���������У�i��2NO(g)+H2(g)=N2(g)+H2O2(1)(����Ӧ)��ii
A.H2O2�Ǹ÷�Ӧ�Ĵ��� B.��Ӧi�Ļ�ܽϸ�
C.�ܷ�Ӧ�����ɷ�Ӧii�����ʾ��� D.��Ӧi��NO��H2����ײ��������Ч
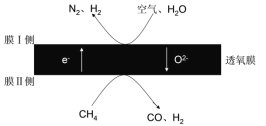
��4��2018���ҹ�ij�����Ŷ�������Ĥ��һ������úϳɰ�ԭ�Ϻͺϳ�Һ̬ȼ�ϵ�ԭ�ϡ��乤��ԭ����ͼ��ʾ��������N2��O2�����ʵ���֮�Ȱ�4��1�ƣ������������У�ĤI������![]() =3����ĤI��ĵ缫����ʽΪ________��
=3����ĤI��ĵ缫����ʽΪ________��