��Ŀ����
11������������ʳ��������������ũҵ�Ի�ѧ���ϵ�������Խ��Խ�����е���������������һ�ֻ��ʣ������ĺϳ�Ϊ���ʵ�������ҵ�춨�˻�������ԭ��Ϊ��N2+3H2?2NH3
��1����N2+3H2?2NH3�ķ�Ӧ�У�һ��ʱ���NH3��Ũ��������0.9mol•L-1����N2��ʾ�䷴Ӧ����Ϊ0.15mol•L-1•s-1������������ʱ��ΪB��
A��2s B��3s C��4s D��6s
��2������4���������ڲ�ͬ�����²�õĺϳɰ���Ӧ�����ʣ����з�Ӧ������B��
A��v��H2��=0.1mol•L-1•min-1
B��v��N2��=0.1mol•L-1•min-1
C��v��NH3��=0.15mol•L-1•min-1
D��v��N2��=0.002mol•L-1•min-1
��3����һ�����º��ݵ��ܱ������У����淴ӦN2��g��+3H2��g��?2NH3��g����H��0�ﵽƽ��ı�־�Ǣڢۢޢࣨ���ţ�
�ٷ�Ӧ����v��N2����v��H2����v��NH3��=1��3��2
�ڸ���ֵ����ʵ���Ũ�Ȳ��ٸı����ϵ��ѹǿ���ٷ����仯
�ܻ��������ܶȲ���
�ݵ�λʱ��������n mol N2��ͬʱ������3n mol H2
��2V��N2����=V��NH3�棩
�ߵ�λʱ����3mol H-H�����ѵ�ͬʱ2mol N-H��Ҳ����
���������ƽ����Է����������ٸı䣮
���� ��1����������֮�ȵ��ڻ�ѧ������֮�ȣ�����N2��ʾ��ƽ����Ӧ����v��N2����������v��NH3�����ٸ������ʶ�����㷴Ӧʱ�䣻
��2��ͬһ��ѧ��Ӧ�У�ͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ����������֮�ȣ��ȰѲ�ͬ���ʵķ�Ӧ���ʻ����ͬһ���ʵķ�Ӧ���ʽ��бȽϣ��Ӷ�ȷ��ѡ�ע�ⵥλ�Ƿ���ͬ��
��3������Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�ȡ��ٷֺ������䣬�Լ��ɴ�������һЩ��Ҳ�������仯������ʱҪע�⣬ѡ���жϵ������������ŷ�Ӧ�Ľ��з����仯�������������ɱ仯����ֵʱ��˵�����淴Ӧ����ƽ��״̬��
��� �⣺��1������v��N2��=0.15mol/��L•s��������֮�ȵ��ڻ�ѧ������֮�ȣ�����v��NH3��=2v��N2��=2��0.15mol/��L•s��=0.3mol/��L•s����
���Է�Ӧ��������ʱ��Ϊ$\frac{0.9mol/L}{0.3mol/��L•s��}$=3s��
�ʴ�Ϊ��B��
��2����ӦΪN2��g��+3H2��g�� 2NH3��g�����������ķ�Ӧ����Ϊ�������жϣ�
2NH3��g�����������ķ�Ӧ����Ϊ�������жϣ�
A���ͣ�H2��=0.1mol/��L•min����
B����N2��=0.1mol/��L•min������Ӧ����֮�ȵ����������֮�ȣ����Ԧͣ�H2��=0.3mol/��L•min����
C��V��NH3��=0.15mol/��L•min������Ӧ����֮�ȵ����������֮�ȣ����Ԧͣ�H2��=0.225mol/��L•min����
D���ͣ�N2��=0.002mol/��L•min������Ӧ����֮�ȵ����������֮�ȣ����Ԧͣ�H2��=0.003mol/��L•min����
���Է�Ӧ����������B��
�ʴ�Ϊ��B��
��3���ٷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ������Ƿ�ﵽƽ��״̬�����ڷ�Ӧ����v��N2����v��H2����v��NH3��=1��3��2�����������ж��Ƿ��ƻ����ʴ���
�ڸ���ֵ����ʵ���Ũ�Ȳ��ٸı䣬��˵�����淴Ӧ������ȣ��ﵽ�ƻ�״̬������ȷ��
����ϵ��ѹǿ���ٷ����仯��˵�����淴Ӧ������ȣ��ﵽ�ƻ�״̬������ȷ��
����������������䣬������������䣬�������Ƿ�ﵽ�ƻ������������ܶȶ����䣬�ʴ���
�ݵ�λʱ��������n mol N2��ͬʱ������3n mol H2����Ϊ�淴Ӧ������˵�����淴Ӧ������ȣ��ʴ���
��2V��N2����=V��NH3�棩����˵�����淴Ӧ������ȣ��ﵽ�ƻ�״̬������ȷ��
�ߵ�λʱ����3mol H-H�����ѵ�ͬʱ2mol N-H��Ҳ���ѣ����淴Ӧ���ʲ��ȣ�û�дﵽ�ƻ�״̬���ʴ���
���������ƽ����Է����������ٸı䣬˵������������ʵ������䣬��˵���ﵽ�ƻ�״̬������ȷ��
�ʴ�Ϊ���ڢۢޢ࣮
���� ���⿼���Ϊ�ۺϣ��漰��ѧƽ��״̬���жϡ����ʵļ����Լ���С�ıȽϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������������Ŀ��飬����ʱ��ע���ж�ƽ��״̬�ı�־�ͽǶȣ�

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�| A�� | pH=4.5�ķ���֭��c��H+����pH=6.5��ţ����c��H+����100�� | |
| B�� | pH��ͬ������ʹ�����Һ����ˮϡ��100��������ҺpH����ͬ | |
| C�� | �к�pH���������ͬ������ʹ�����Һ������NaOH�����ʵ�����ͬ | |
| D�� | 25�棬��pH=5������ϡ��1000������Һ��pH=8 |
| A�� | ������������ˮ�й��� | |
| B�� | ��������Ͷ���Ȼ�����Һ�� | |
| C�� | ��������ˮ�����Ȼ�����Һ�� | |
| D�� | ����������������Һ�����Ȼ�����Һ�� |
| A�� | x��ֵΪ1 | |
| B�� | ��B��Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ0.2 mol•L-1•min-1 | |
| C�� | ��Ӧ��ʼǰA�����ʵ���Ϊ3 mol | |
| D�� | 5 minʱA��Ũ��Ϊ0.2 mol•L-1 |
| A�� | ����2molSO3 | |
| B�� | SO2��SO3���ʵ���֮��һ��Ϊ2mol | |
| C�� | �ų�197kJ������ | |
| D�� | ƽ��ʱ����������ѹǿ�Ϳ�ʼʱѹǿ��� |
| A�� | 1mol/L��NaOH��Һ��Na+�����ʵ���Ϊ1mol | |
| B�� | ���³�ѹ�£�32gO2��O3�Ļ�������к��е�ԭ������Ϊ2NA | |
| C�� | 0.1mol��������������������Ӧ��ת�Ƶĵ���Ϊ0.3NA | |
| D�� | 25��ʱ��l L pH=13��Ba��OH��2��Һ�к���OH-����ĿΪ0.2NA |
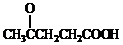 ���ϳɾ۷���E��·�ߣ�
���ϳɾ۷���E��·�ߣ�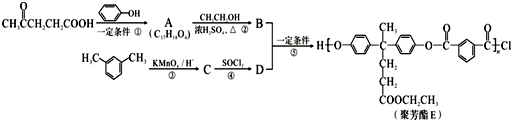
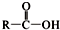 +SOCl2��
+SOCl2��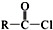 +SO2+HCl
+SO2+HCl +R��OH��
+R��OH��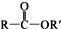 +HCl ��R��R���ʾ������
+HCl ��R��R���ʾ������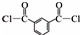 ��
��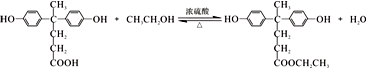 ��
�� �ṹ
�ṹ ��
��