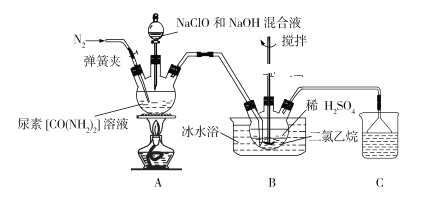
��Ŀ����
����Ŀ���밴Ҫ����գ�
��1����![]() ��������___��
��������___��
��![]() ��������___��
��������___��
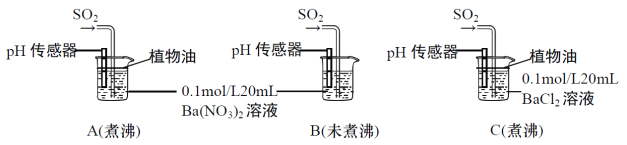
�۱�����Ũ��ˮ��Ӧ�Ļ�ѧ����ʽ��___��
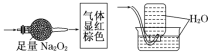
�ܼ�ȩ������������ͭ����Һ��Ӧ�Ļ�ѧ����ʽ��___��
��ij�л�������A�ķ���ʽΪC5H11Br�����ӽṹ����������CH3������![]() ��һ����Br����A�Ľṹ��ʽΪ___��
��һ����Br����A�Ľṹ��ʽΪ___��
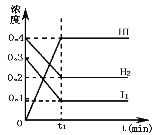
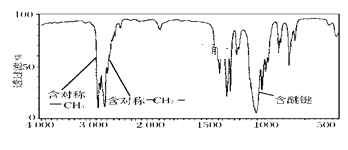
��2��ij�л���A����Է�������Ϊ74���Һ������ͼ��ͼ����A�Ľṹ��ʽΪ__��
��3��1���ij����������ȫȼ�����ɵ�CO2�����ɵ�ˮ������1�������ͬ��ͬѹ�²ⶨ����0.1mol������ȫȼ�յIJ��ﱻ��ʯ�����գ���ʯ������39g�������ķ���ʽΪ__����������һ�ȴ�����3�֣�д���������п��ܵĽṹ��ʽ__��
���𰸡�3-��-2-���� 2-��-2��4-����ϩ ![]() +3Br2
+3Br2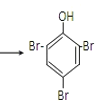 ��+3HBr HCHO+4Cu(OH)2+2NaOH
��+3HBr HCHO+4Cu(OH)2+2NaOH![]() 2Cu2O��+Na2CO3+6H2O CH3CHBrCH(CH3)2 CH3CH2OCH2CH3 C6H14 CH3(CH2)4CH3��(CH3)3CCH2CH3
2Cu2O��+Na2CO3+6H2O CH3CHBrCH(CH3)2 CH3CH2OCH2CH3 C6H14 CH3(CH2)4CH3��(CH3)3CCH2CH3
��������
��1���ٸ��ݴ������������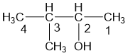 ������Ϊ��3-��-2-������
��������3-��-2-������
�ڸ��ݶ�ϩ������������![]() ������Ϊ��2-��-2��4-����ϩ��
������Ϊ��2-��-2��4-����ϩ��
�۱�����Ũ��ˮ����ȡ����Ӧ��Br2ȡ�����ӷ����ϵ�������λ��һ����λ����Ӧ�Ļ�ѧ����ʽΪ��![]() +3Br2
+3Br2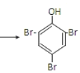 ��+3HBr��
��+3HBr��
�ܼ�ȩ������������ͭ����Һ����������Ӧ������ש��ɫ��Cu2O���������ڼ�ȩ��2��ȩ����������4������Cu(OH)2��Ӧ����ѧ����ʽΪ��HCHO+4Cu(OH)2+2NaOH![]() 2Cu2O��+Na2CO3+6H2O��
2Cu2O��+Na2CO3+6H2O��
�ݸ������⣬ij�л�������A�ķ���ʽΪC5H11Br�����ӽṹ����������CH3������![]() ��һ����Br��A�Ľṹ��ʽΪ��CH3CHBrCH(CH3)2��
��һ����Br��A�Ľṹ��ʽΪ��CH3CHBrCH(CH3)2��
��2�����л�������ԭ������Ϊ74���������ͼ��ʾ���жԳƵļ����ԳƵ��Ǽ����Ѽ�������л���Ľṹ��ʽΪCH3CH2OCH2CH3��
��3�������ķ���ʽΪCxHy��ȼ�շ���ʽΪ��
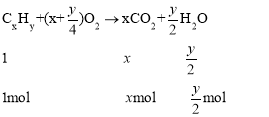
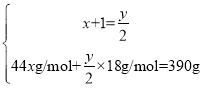
���![]() �����Ը�������ʽΪC6H14�����ڸ�����һ�ȴ��������֣��������ֲ�ͬ������H������ṹ��ʽΪCH3(CH2)4CH3��(CH3)3CCH2CH3��
�����Ը�������ʽΪC6H14�����ڸ�����һ�ȴ��������֣��������ֲ�ͬ������H������ṹ��ʽΪCH3(CH2)4CH3��(CH3)3CCH2CH3��

 ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�