��Ŀ����
14������A��B��C��D��E����Ԫ�أ����ǵ��������������࣮��A�ĺ˵��������2�����γ��⻯��H2A�����⻯���ڳ�������Һ�壻
��A��B��Ԫ�ؿ��γ�B2A3������û������������ǿ�ᣬ��������ǿ�
��C+��B3+��8�����ӣ�
��C��DԪ�ؿ����γɻ�����CD��
��CD����Һ��ͨ��������ӵ�����Һ����ɫ��
�������ڱ���E����C�����������ڣ�E���ʿ�����ˮ��Ӧ������������ӦʱE�ĵ��������ɵ����������ʵ���֮��Ϊ2��1���Իش�
��1��E��Cs��дԪ�ط��ţ���
��2��B�����ӽṹʾ��ͼ
 ��CԪ������������Ӧ��ˮ����ĵ���ʽ
��CԪ������������Ӧ��ˮ����ĵ���ʽ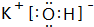 ��
����3���õ���ʽ��ʾH2A�γɹ��̣�
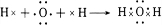 ��
����4��д��CD����Һ��ͨ�����������ӷ���ʽ��Cl2+2I-�T2Cl-+I2��
��5���Ƚ�B��C��E����Ԫ���γɵļ����������Ե�ǿ������B��C��E������ʵ�����ӷ��ű�ʾ�������ԣ�Al3+��K+��Cs+��
���� ����A��B��C��D��E����Ԫ�أ����ǵ��������������࣮A�ĺ˵��������2�����γ��⻯��H2A�����⻯��ij�������Һ�壬��AΪOԪ�أ��⻯��ΪH2O��A��B��Ԫ�ؿ��γ�B2A3������û������������ǿ�ᣬ��������ǿ���BΪAl���γɵĻ�����ΪAl2O3��C+���ӱ�B2+���Ӷ�8�����ӣ���CΪKԪ�أ�C��DԪ�ؿ����γɻ�����CD����CD����Һ��ͨ��������ӵ�����Һ����ɫ����DΪIԪ�أ������ڱ���E����C�����������ڣ�E���ʿ�����ˮ��Ӧ������������ӦʱE�ĵ��������ɵ����������ʵ���֮��Ϊ2��1��E����+1�ۣ���EΪCs���ݴ˽��
��� �⣺����A��B��C��D��E����Ԫ�أ����ǵ��������������࣮A�ĺ˵��������2�����γ��⻯��H2A�����⻯��ij�������Һ�壬��AΪOԪ�أ��⻯��ΪH2O��A��B��Ԫ�ؿ��γ�B2A3������û������������ǿ�ᣬ��������ǿ���BΪAl���γɵĻ�����ΪAl2O3��C+���ӱ�B2+���Ӷ�8�����ӣ���CΪKԪ�أ�C��DԪ�ؿ����γɻ�����CD����CD����Һ��ͨ��������ӵ�����Һ����ɫ����DΪIԪ�أ������ڱ���E����C�����������ڣ�E���ʿ�����ˮ��Ӧ������������ӦʱE�ĵ��������ɵ����������ʵ���֮��Ϊ2��1��E����+1�ۣ���EΪCs��
��1��E��CsԪ�أ��ʴ�Ϊ��Cs��
��2��B������ΪAl3+���ṹʾ��ͼΪ ��CԪ������������Ӧ��ˮ����ΪKOH������ʽ��
��CԪ������������Ӧ��ˮ����ΪKOH������ʽ��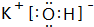 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��3���õ���ʽ��ʾH2O�γɹ��̣�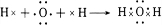 ��
��
�ʴ�Ϊ��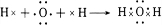 ��
��
��4��KI����Һ��ͨ�����������ӷ���ʽ��Cl2+2I-�T2Cl-+I2���ʴ�Ϊ��Cl2+2I-�T2Cl-+I2��
��5��������Cs��K��Al���ʼ����������Ե�ǿ����Al3+��K+��Cs+���ʴ�Ϊ��Al3+��K+��Cs+��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ������ضԻ�ѧ����Ŀ��飬ע��Ի���֪ʶ���������գ�

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�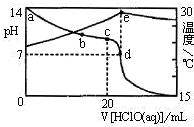 NaClO������ˮ�ľ�����ֽ��Ư�ס������ȣ�������ҽҩ��ҵ���������Ȱ���
NaClO������ˮ�ľ�����ֽ��Ư�ס������ȣ�������ҽҩ��ҵ���������Ȱ�����1��NaClO��Һ��ɱ��������ԭ��֮һ������Һ�д�����Ч�ȣ�HClO����д��NaClOˮ������ӷ���ʽ��ClO-+H2O
 HClO+OH-��
HClO+OH-����2��NaClO��Һ��ɱ��Ч������Һ��Ũ�ȡ��¶ȼ���Һ�д��ڵ����йأ�
�ٲ�ͬ�¶��£�500mL 0.1mol?L-1NaClO��Һ��1m3�ռ��ɱ��Ч�����±���ʾ��
| �¶�/�� | 20 | 30 | 40 | 80 |
| ʱ��/min | 10 | 10 | 10 | 10 |
| ɱ���� | 83% | 90% | 97% | 92% |
�������������NaClO��Һ����Ч������cd������ĸ����
a��NH4+ b��ClO- c��OH- d��SO32-
��3�������£���1.00mol?L-1��HClO����20.00mL 1.00mol?L-1NaOH��Һ�У���Һ��pH���¶������HClO����ı仯������ͼ��ʾ��
��a��b��c��d�ĵ��У�ˮ�ĵ���̶�������c������ĸ����
������˵������ȷ����AB������ĸ����
A��a��b��c��d�ĵ���Һ��d����Һ��ClO-�����ʵ������
B��c����Һ�У�c��Na+����c��ClO-����c��OH-����c��H+��
C��d����Һ�У�c��Na+��=c��ClO-��+c��HClO��
D��e�����Һ�¶��½�����Ҫԭ����HClO�������ȣ�
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ԭ�Ӱ뾶��W��Y��Z��X | |
| B�� | ����������Ӧˮ��������ԣ�Y��W | |
| C�� | ���ʷе�W��Z | |
| D�� | X��Y��Z���ܴ�����ͬһ���ӻ������� |
| A�� | �ʱ���һ����Ӧ�ܷ��Է�������ص����أ��������ȷ�Ӧ���Է����� | |
| B�� | һ����Ӧ�ܷ��Է����У����ʱ���ر�Ĺ�ͬӰ���й� | |
| C�� | ��ͬһ�����²�ͬ�����в�ͬ����ֵ������ϵ�Ļ��ҳ̶�Խ����ֵԽ�� | |
| D�� | �Է����еķ�Ӧһ����Ѹ�ٽ��� |
| A�� | ���������绯��ʴ��������Ӧʽ��Fe-2e-=Fe2+ | |
| B�� | ���������¼���ȼ�ϵ�صĸ�����Ӧʽ��CH4+2H2O-8e-=CO2��+8H+ | |
| C�� | ��ͭ����ʱ�����Դ�����������Ǵ�ͭ | |
| D�� | �û��Ե缫��ⱥ��ʳ��ˮ�������ĵ缫��ӦʽΪ��2Cl--2e-=Cl2�� |
| A�� | ��λ����ȡ������ֻ��һ�� | |
| B�� | ��λ����ȡ������ֻ��һ�� | |
| C�� | ��λ����ȡ������ֻ��һ�� | |
| D�� | �����ܷ���ȡ����ӦҲ�ܷ����ӳɷ�Ӧ |