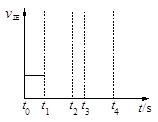��Ŀ����
��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2(g)��3H2(g) 2NH3(g) ��H��0 ����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ��ʾ����ش��������⣺
2NH3(g) ��H��0 ����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ��ʾ����ش��������⣺
����ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v(NH3)= ��
��������������˵��������Ӧ�Ѵ�ƽ����� ��
| A��3v����H2����2v����NH3�� |
| B�������������ƽ����Է���������ʱ����仯 |
| C��������������ܶȲ���ʱ����仯 |
| D������������ķ�����������ʱ����仯 |
��1��0.025mol/(L��min)(2�֣�©д��λ��1�֣�
��2��BD��2�֣�
��3������2�֣�
���������������1����ͼ�жϷ�Ӧ��4����ʱ��Ӧ�ﵽƽ�⣬v(NH3)=0.1��4=0.025mol/(L��min)����2��
A�������ϻ�ѧ��������ϵ������B�������������ƽ����Է�������һ�����ϱ仯������������ʱ���ﵽƽ�⣬��ȷ��C��������������䣬�ܶ���һ������������D�����Ӹ�����һ��������������ʱ���ﵽƽ�⣬��ȷ����3���ı��������ƽ����ԭƽ����ǵ�Чƽ�⣬��ƽ�ⲻ�ƶ���
���㣺���黯ѧƽ��Ľ������ƶ����й����⡣

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д���֪�ظ����(K2Cr2O7)��һ����Ҫ�ĵ����Լ���
��1��ǿ�����Ե�K2Cr2O7��Һ�У���������ƽ�⣺ Cr2O72-����ɫ����H2O 2CrO42-����ɫ����2H+��
2CrO42-����ɫ����2H+��
����Ҫʹ��Һ�ij�ɫ������пɲ��õķ����� ��
| A����������ϡ���� | B����������ϡ���� | C�����������ռ���� | D����ˮϡ�͡� |
ijѧ��Ϊ��̽��п�����ᷴӦ���������ʱ仯����100mLϡ�����м���������п�ۣ���״���²�������ۼ�ֵ���£�
| ʱ�䣨min�� | 1 | 2 | 3 | 4 | 5 |
| ���������mL�� | 50 | 120 | 232 | 290 | 310 |
��1����0��1��1��2��2��3��3��4��4��5 minʱ����У���Ӧ��������ʱ����� ��
ԭ��Ϊ ����Ӧ������С��ʱ����� ��
ԭ��Ϊ ����2����2��3minʱ����ڣ��������Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ ��
��3��Ϊ�˼�����Ӧ���ʵ������ٲ��������������������зֱ����������������Һ��A .����ˮ B .Na2SO4��Һ C. NaNO3��Һ D. CuSO4��Һ E. Na2CO3 ��Һ, ����Ϊ���е��� ��
��֪A(g)+B(g) C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
C(g)+D(g)��Ӧ��ƽ�ⳣ�����¶ȵĹ�ϵ���£�
| �¶�/ �� | 700 | 800 | 830 | 1000 | 1200 |
| ƽ�ⳣ�� | 1.7 | 1.1 | 1.0 | 0.6 | 0.4 |
�ش��������⣺
��1���÷�Ӧ��ƽ�ⳣ������ʽK= ����H 0���<���� >���� =��)��
��2��830��ʱ����һ��5 L���ܱ������г���0.20mol��A��0.80mol��B���練Ӧ��ʼ6s��A��ƽ����Ӧ����v(A)="0.003" mol��L-1��s-1����6sʱc(A)= mol��L-1�� C�����ʵ���Ϊ mol������Ӧ��һ��ʱ��ﵽƽ��ʱA��ת����Ϊ �������ʱ����ܱ��������ٳ���1 mol�����ƽ��ʱA��ת����Ϊ ��
��3���жϸ÷�Ӧ�Ƿ�ﵽƽ�������Ϊ (����ȷѡ��ǰ����ĸ)��
a��ѹǿ����ʱ��ı� b��������ܶȲ���ʱ��ı�
c��c(A)����ʱ�ʸı� d����λʱ��������C��D�����ʵ������
��4��1200��ʱ��ӦC(g)+D(g)
 A(g)+B(g)��ƽ�ⳣ����ֵΪ ��
A(g)+B(g)��ƽ�ⳣ����ֵΪ �� �״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2O Ϊԭ�ϣ�ͨ����ӦI�͢����Ʊ��״���
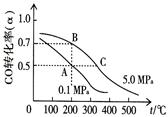
�Ž�1.0 mol CH4��2.0 mol H2O(g)ͨ�˷�Ӧ��(�ݻ�Ϊ100L)����һ�������·�����Ӧ:
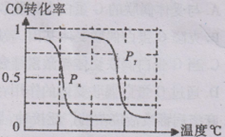
CH4(g)��H2O(g) CO(g)��3H2(g)����I��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CO(g)��3H2(g)����I��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ ��
��ͼ�е�P1_ P2(�<������>����=��),100��ʱƽ�ⳣ��Ϊ ��
�۸÷�Ӧ�ġ�H 0(�<������>����=��)��
(2)��һ�������£���a mol CO��3a mol H2�Ļ�������ڴ������������Է���Ӧ���ɼ״�: CO(g)+2H2(g) CH3OH(g) ��H<0 ������
CH3OH(g) ��H<0 ������
���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ���
A�������¶� B����CH3OH(g)����ϵ�з���
C������He��ʹ��ϵ��ѹǿ���� D���ٳ���lmol CO��3 mol H2
��Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С�
A���±���ʣ���ʵ����������: a=_ ��b=_ ��
| ʵ���� | T���棩 | n(CO)��n(H2) | P(Mpa) |
| 1 | 150 | 1��3 | 0.1 |
| 2 | a | 1��3 | 5 |
| 3 | 350 | b | 5 |
��14�֣�I����֪��C(s)��H2O(g) CO(g)��H2(g) ��H
CO(g)��H2(g) ��H
һ���¶��£���1.0 L�ܱ������з���1 mol C��s����1 mol H2O(g)���з�Ӧ,��Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±���
| ʱ��t/h | 0 | 1 | 2 | 4 | 8 | 16 | 20 | 25 | 30 |
| ��ѹǿp/100 kPa | 4.56 | 5.14 | 5.87 | 6.30 | 7.24 | 8.16 | 8.18 | 8.20 | 8.20 |
��1��������Щѡ�����˵���ÿ��淴Ӧ�Ѵ�ƽ��״̬ ��
A�����������ܶȲ��ٷ����ı� B������1 mol H2O��g����ͬʱ����1 mol H2
C����H���� D��v��(CO) = v��(H2)
��2������ѹǿP����ʼѹǿP0��ʾ��Ӧ��ϵ�������ʵ���n����n����____ mol���ɱ������ݼ��㷴Ӧ��ƽ��ʱ����Ӧ��H2O(g)��ת���ʦ� =_____����ȷ��С�����ڶ�λ����
�����ʼ��仯�����ڹ�ũҵ������������Ҫ��Ӧ�á�
��1����֪25��ʱ��xSO2 (g)��2xCO(g)��2xCO2 (g)��Sx (s) ��H��ax kJ/mol ��
2xCOS(g)��xSO2 (g)��2xCO2 (g)��3Sx (s) ��H��bx kJ/mol�� ��
��ӦCOS(g)����CO(g)��Sx (s)���Ȼ�ѧ����ʽ�� ��
��2��������ʵ���Ũ��Na2S��NaOH�����Һ�еμ�ϡ����������������H2S��HS?��S2?�ķֲ�������ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ�������μ���������Ĺ�ϵ��ͼ��ʾ�����Եμӹ���H2S������ݳ������Է�����
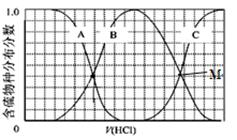
��B���ߴ��� �����仯(�������ű�ʾ)���μӹ����У���Һ��һ��������
c(Na+)= ��
��M�㣬��Һ����Ҫ�漰�����ӷ���ʽ ��
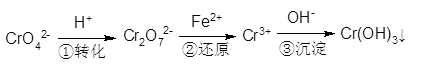
 Cr2O72-(��ɫ)+H2O
Cr2O72-(��ɫ)+H2O Cr3+(aq)+3OH��(aq)
Cr3+(aq)+3OH��(aq)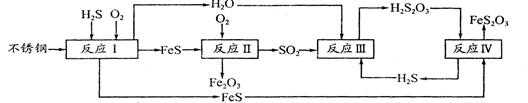
 H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��
H2S(g) ��H=��21��6kJ��mol��1����Ӧ�ﵽƽ��ʱH2��S��H2S�����ʵ�����Ϊ3 mol����380 Kʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��Ϊ______�����жԸ÷�Ӧ������ȷ����______(����ĸ���)��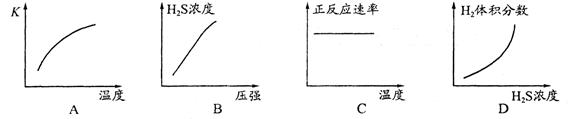
 CO(g)+H2(g)?131.4 kJ��
CO(g)+H2(g)?131.4 kJ��