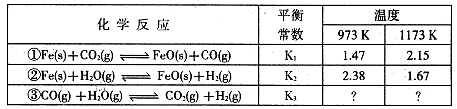
��Ŀ����
�״���һ�ֺܺõ�ȼ�ϣ���ҵ����CH4��H2O Ϊԭ�ϣ�ͨ����ӦI�͢����Ʊ��״���
�Ž�1.0 mol CH4��2.0 mol H2O(g)ͨ�˷�Ӧ��(�ݻ�Ϊ100L)����һ�������·�����Ӧ:
CH4(g)��H2O(g) CO(g)��3H2(g)����I��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
CO(g)��3H2(g)����I��CH4��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
����֪100��ʱ�ﵽƽ�������ʱ��Ϊ5min������H2��ʾ��ƽ����Ӧ����Ϊ ��
��ͼ�е�P1_ P2(�<������>����=��),100��ʱƽ�ⳣ��Ϊ ��
�۸÷�Ӧ�ġ�H 0(�<������>����=��)��
(2)��һ�������£���a mol CO��3a mol H2�Ļ�������ڴ������������Է���Ӧ���ɼ״�: CO(g)+2H2(g) CH3OH(g) ��H<0 ������
CH3OH(g) ��H<0 ������
���������ݻ����䣬���д�ʩ�����Ӽ״����ʵ���
A�������¶� B����CH3OH(g)����ϵ�з���
C������He��ʹ��ϵ��ѹǿ���� D���ٳ���lmol CO��3 mol H2
��Ϊ��Ѱ�Һϳɼ״����¶Ⱥ�ѹǿ������������ijͬѧ���������ʵ�飬����ʵ�������Ѿ���������ʵ����Ʊ��С�
A���±���ʣ���ʵ����������: a=_ ��b=_ ��
| ʵ���� | T���棩 | n(CO)��n(H2) | P(Mpa) |
| 1 | 150 | 1��3 | 0.1 |
| 2 | a | 1��3 | 5 |
| 3 | 350 | b | 5 |
��14�֣���1����0.0030mol/(L��min) ��2�֣� �ڣ� ��2�֣�
2.25��10��4(mol/L)2��2�֣���д��λ���۷֣���λд����1�֣� �ۣ���2�֣�
��2����BD��2�֣� ��A.a��150��b��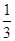 ����1�֣���2�֣� B.0.1��2�֣�
����1�֣���2�֣� B.0.1��2�֣�
���������������1������ͼ���֪��ƽ��ʱ�����ת����Ϊ0.5�����Լ�������ʵ���������1.5mol��0.5��0.5mol�����c(CH4)��0.5mol��100L��0.005mol/L������v(CH4)��0.005mol/L��5min��0.001mol/��L?min��������֮�ȵ��ڻ�ѧ������֮�ȣ�����v(H2)��3v(CH4)��3��0.001mol/��L?min��=0.003mol/��L?min����
���¶���ͬʱ������ֱx��ĸ����ߣ�����ѹǿΪP1��CH4��ת���ʸߣ���ӦΪǰ���������ķ�Ӧ��ѹǿ����ƽ���������С�ķ����ƶ��������淴Ӧ�ƶ���CH4��ת���ʽ��ͣ�����P1��P2����
CH4��g���� H2O��g�� CO��g���� 3H2��g��
CO��g���� 3H2��g��
��ʼŨ�ȣ�mol/L�� 0.010 0.020 0 0
ת��Ũ�ȣ�mol/L�� 0.005 0.005 0.005 0.015
ƽ��Ũ�ȣ�mol/L�� 0.005 0.015 0.005 0.015
����ƽ�ⳣ��K��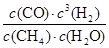 =
=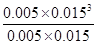 ��2.25��10-4��
��2.25��10-4��
����ͼ��֪���¶�Խ�����ת����Խ�������¶�ƽ��������Ӧ�����ƣ��¶�����ƽ�������ȷ����ƶ�����������ӦΪ���ȷ�Ӧ������H��0��
��2����A���÷�ӦΪ���ȷ�Ӧ�������¶ȣ�ƽ�������ȷ����ƶ��������淴Ӧ�����ƶ����״��IJ��ʽ��ͣ���A����B����CH3OH��g������ϵ�з��룬�����Ũ�Ƚ��ͣ�ƽ��������Ӧ�ƶ����״��IJ������ӣ���B����C������He��ʹ��ϵ��ѹǿ���������ݻ����䣬��Ӧ���������Ũ�Ȳ��䣬ƽ�ⲻ�ƶ����״��IJ��ʲ��䣬��C����D���ٳ���1molCO��3molH2���ɵ�ЧΪѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�����ƶ����״��IJ������ӣ���D��ȷ����ѡBD��
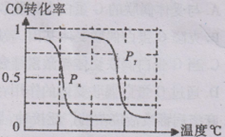
��A����ȡ���Ʊ�������̽���ϳɼ״����¶Ⱥ�ѹǿ�����������������¶ȡ�ѹǿ�DZ仯�ģ�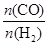 Ӧ���ֲ��䣬����b��
Ӧ���ֲ��䣬����b�� ���Ƚ�ʵ��1��2��֪��ѹǿ��ͬ�������¶�Ӧ��ͬ����a��150��
���Ƚ�ʵ��1��2��֪��ѹǿ��ͬ�������¶�Ӧ��ͬ����a��150��
B���¶���ͬʱ������ֱx��ĸ����ߣ�����ѹǿΪPy��CO��ת���ʸߣ���ӦΪǰ�������С�ķ�Ӧ��ѹǿ����ƽ���������С�ķ����ƶ�����������Ӧ�ƶ�������Px��Py�����ѹǿPx��0.1Mpa��
���㣺���鷴Ӧ���ʡ�ƽ�ⳣ���ļ��㣻���������ƽ��״̬��Ӱ���Լ�ͼ��ʶ���

�о�NO2����CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
���÷�Ӧ6NO2(g)��8NH3(g)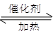 7N2(g)��12 H2O(g)�ɴ���NO2��700��ʱ�����ݻ�Ϊ2L���ܱ������г���һ������NO2��NH3, ��Ӧ�����вⶨ�IJ������ݼ��±�
7N2(g)��12 H2O(g)�ɴ���NO2��700��ʱ�����ݻ�Ϊ2L���ܱ������г���һ������NO2��NH3, ��Ӧ�����вⶨ�IJ������ݼ��±�
| ��Ӧʱ��/min | n(NO2)/mol | n(NH3)/ mol |
| 0 | 1��20 | 1��60 |
| 2 | 0��90 | |
| 4 | | 0��40 |
��2��700�� ����NO2��NH3�������1:2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
a����ϵѹǿ���ֲ���
b�����������ɫ���ֲ���
c����ϵ�ܶȱ��ֲ���
d��ÿ����1 mol NO2��ͬʱ����2 mol H2O
(12��)�����Ϊ2L�Ĺ̶��ܱ�������ͨ��3molX����,��һ���¶��·������·�Ӧ��
2X(g) Y(g)+3Z(g)
Y(g)+3Z(g)
��1����5min��Ӧ�ﵽƽ��,��ʱ��������ڵ�ѹǿΪ��ʼʱ��1.2��,����Y�����ʵ���Ũ�ȱ仯��ʾ������Ϊ ��
��2����������Ӧ�ڼס��ҡ��������ĸ�ͬ�����ܱ������н���,��ͬһ��ʱ���ڲ�������ڵķ�Ӧ���ʷֱ�Ϊ����v(X)��3.5mol/(L?min)����v(Y)��2mol/(L?min)����v(Z)=4.5mol/(L?min)����v(X)��0.075mol/(L?s)��������������ͬ,�¶Ȳ�ͬ�����¶��ɸߵ��͵�˳����(�����) ��
��3������ﵽ(1)��ƽ����ϵ�г������,��ƽ���� (��"��"��"��"��"��")�ƶ�������
�ﵽ(1)��ƽ����ϵ�����߲��ֻ������,��ƽ���� (��" �� " �� " �� " �� " ��")�ƶ���
��4��������ͬ��������ﵽ(1)������ƽ����ϵ���ٳ���0.5molX����,��ƽ���X��ת���ʢ���ŵ�
ƽ���е�X��ת������Ƚ� ��
| A����ȷ�� | B����һ�����ڢ� | C����һ�����ڢ� | D����һ��С�ڢ� |
��(1)��ƽ���Ч,��a��b��cӦ������Ĺ�ϵΪ ��
��6���������¶Ⱥ�������䣬��ʼʱ����X��Y��Z���ʵ����ֱ�Ϊamol��bmol��cmol,�ﵽƽ��ʱ��
��(1)��ƽ���Ч,����ʼʱά�ֻ�ѧ��Ӧ���淴Ӧ�������,��c��ȡֵ��ΧӦ��Ϊ ��
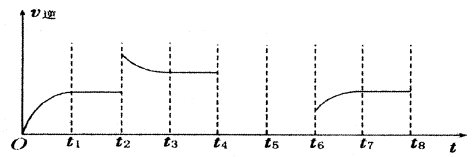
��һ�̶��ݻ�Ϊ2L���ܱ������ڼ���0.2mol��N2��0.6mol��H2����һ�������·������·�Ӧ��N2(g)��3H2(g) 2NH3(g) ��H��0 ����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ��ʾ����ش��������⣺
2NH3(g) ��H��0 ����Ӧ��NH3�����ʵ���Ũ�ȵı仯�������ͼ��ʾ����ش��������⣺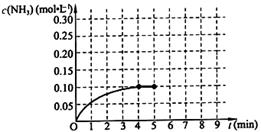
����ͼ������ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v(NH3)= ��
��������������˵��������Ӧ�Ѵ�ƽ����� ��
| A��3v����H2����2v����NH3�� |
| B�������������ƽ����Է���������ʱ����仯 |
| C��������������ܶȲ���ʱ����仯 |
| D������������ķ�����������ʱ����仯 |
��1���̶�������CO2����Ч��������Դ�������ٿ����е��������塣��ҵ����һ����CO2�������״�ȼ�ϵķ�����CO2(g)��3H2(g) CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��
CH3OH(g)��H2O(g)��H����49��0kJ��mol��1��ij��ѧʵ�齫6molCO2��8molH2����2L�ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���ͼ������a��1��6����������˼�ǣ���l minʱH2�����ʵ�����6mol��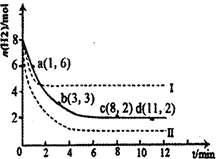
������ʱ���ƽ����Ӧ����������__________����С����______________��
| A��0��1min | B��1��3min | C��3��8min | D��8��11min |
��2�����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ��������CO2��H2O(g)�ڲ�ͬ����(��)�����£�CH4���������ʱ��ı仯��ͼ��ʾ����0~30 h�ڣ�CH4��ƽ����������v(��)��v(��)��v(��)�Ӵ�С��˳��Ϊ ����Ӧ��ʼ���12Сʱ�ڣ��ڵ�___________�ִ����������£��ռ���CH4��ࡣ

 7N2(g)+12H2O(g)+Q��Q��0����
7N2(g)+12H2O(g)+Q��Q��0���� W (s) + 3H2O (g) ��ش��������⣺
W (s) + 3H2O (g) ��ش��������⣺ WI4 (g)������˵����ȷ����__________��
WI4 (g)������˵����ȷ����__________��