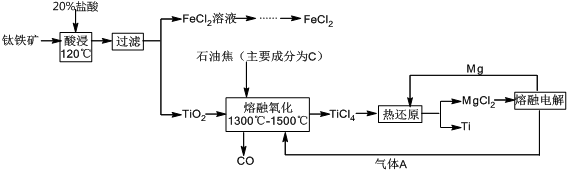
��Ŀ����
ʯ���ͣ���17��̼ԭ�����ϵ�Һ̬���������ֽ�ʵ�鰴����ͼ����
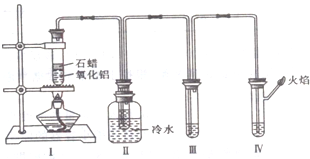
��1��ʯ���ͷֽ�ʵ��������������Ҫ�ɷ��� ����д��ţ���ͬ��
��ֻ�м��顡��ֻ����ϩ����������ϩ���Ļ����
��2��װ�â���ƿ�е���ˮ������ ���Թܵײ��� ������
��3��װ�â�ʢװ��ϡ���������Һ���淴Ӧ���У����Թ۲쵽װ�â���Թ�����Һ��ɫ�ı仯�� ����˵��װ��I�Թܵķ�Ӧ�������� ������
��4��װ�â���ʢװ��������Ȼ�̼��Һ������Һ����ɫ�仯�� ��

��1��ʯ���ͷֽ�ʵ��������������Ҫ�ɷ���
��ֻ�м��顡��ֻ����ϩ����������ϩ���Ļ����
��2��װ�â���ƿ�е���ˮ������
��3��װ�â�ʢװ��ϡ���������Һ���淴Ӧ���У����Թ۲쵽װ�â���Թ�����Һ��ɫ�ı仯��
��4��װ�â���ʢװ��������Ȼ�̼��Һ������Һ����ɫ�仯��
���㣺̽��ʯ���ͷֽ�����ϩ����ϩ�Ļ�ѧ����
ר�⣺ʵ����
��������1��ʯ���ͷֽ�����ϩ���������Ļ����ݴ˽��н��
��2����ˮ��ȴʯ���͵ķֽ������Եõ����͵�ȼ�ͣ����Թܵĵײ�����״�����ɣ�
��3��ʯ���͵ķֽ�����к��в����������ܹ������Ը��������Һ����������ԭ��Ӧ��
��4����������̬���ܹ���������Ȼ�̼�����ӳɷ�Ӧ���Ӷ�����������Ȼ�̼�ĺ���ɫ��ɫ��
��2����ˮ��ȴʯ���͵ķֽ������Եõ����͵�ȼ�ͣ����Թܵĵײ�����״�����ɣ�
��3��ʯ���͵ķֽ�����к��в����������ܹ������Ը��������Һ����������ԭ��Ӧ��
��4����������̬���ܹ���������Ȼ�̼�����ӳɷ�Ӧ���Ӷ�����������Ȼ�̼�ĺ���ɫ��ɫ��
���
�⣺��1��ʯ������Ҫ�Ǻ�17��̼ԭ�����ϵ�Һ̬���������ڼ��������£�ʯ���ͷֽ�����ϩ��������ԭ���غ�֪����������ϩ�������������
�ʴ�Ϊ���ۣ�
��2�������ѻ�������̼ԭ�����ϴ���������ʣ���������ˮ��ȴ�����Եõ����͵�ȼ�ͣ�����װ�â���ƿ�е���ˮ�����ǶԲ����ѻ����������Թܵײ�����״�����ɣ�
�ʴ�Ϊ���Բ����ѻ�����������״�
��3������ʯ�����ѽ�����к��в����������ܹ������Ը��������Һ����������ԭ��Ӧ�������淴Ӧ���У����Թ۲쵽װ�â���Թ�����Һ�Ϻ�ɫ��ȥ��
�ʴ�Ϊ���Ϻ�ɫ��ȥ����ԭ�����壨������̬������
��4��ʯ���ͷֽ�����к��в����͵���̬�����ܹ���������Ȼ�̼�����ӳɷ�Ӧ������װ�â���ʢװ��������Ȼ�̼��Һ��ɫ��
�ʴ�Ϊ������ɫ��ȥ��
�ʴ�Ϊ���ۣ�
��2�������ѻ�������̼ԭ�����ϴ���������ʣ���������ˮ��ȴ�����Եõ����͵�ȼ�ͣ�����װ�â���ƿ�е���ˮ�����ǶԲ����ѻ����������Թܵײ�����״�����ɣ�
�ʴ�Ϊ���Բ����ѻ�����������״�
��3������ʯ�����ѽ�����к��в����������ܹ������Ը��������Һ����������ԭ��Ӧ�������淴Ӧ���У����Թ۲쵽װ�â���Թ�����Һ�Ϻ�ɫ��ȥ��
�ʴ�Ϊ���Ϻ�ɫ��ȥ����ԭ�����壨������̬������
��4��ʯ���ͷֽ�����к��в����͵���̬�����ܹ���������Ȼ�̼�����ӳɷ�Ӧ������װ�â���ʢװ��������Ȼ�̼��Һ��ɫ��
�ʴ�Ϊ������ɫ��ȥ��
���������⿼���˿�����̽��ʯ���͵ķֽ⼰��ϩ�Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ���ȷʯ���ͷֽ���P���鷽������������������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
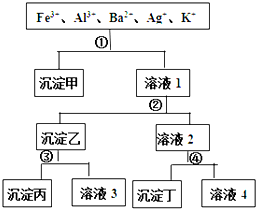 ij��Һ����Fe3+��Al3+��Ba2+��Ag+��K+���������ӣ����ù�����H2SO4��NaOH��NH3?H2O��NaCl������Һ������ͼ��ʾ�IJ���ֿ��������ӣ������ж���ȷ���ǣ�������
ij��Һ����Fe3+��Al3+��Ba2+��Ag+��K+���������ӣ����ù�����H2SO4��NaOH��NH3?H2O��NaCl������Һ������ͼ��ʾ�IJ���ֿ��������ӣ������ж���ȷ���ǣ�������| A�����������������������������Ļ���� |
| B����Һ3�к���Al3+ |
| C����Һ4�������������ӣ��ֱ���H+��Na+��K+ |
| D���Լ�����NaCl���Լ�����H2SO4 |
����ʵ�����������������ǣ�������
| A�����������о���ȼ�գ���������ɫ���� |
| B�����ȵ���˿��������ȼ�գ��������䣬���ɺ�ɫ���� |
| C��SO2ͨ��ʯ���Լ��У���Һ�ȱ�����ɫ |
| D�����ڿ�����ȼ�գ�������ɫ�Ļ��棬���ɵ���ɫ���� |

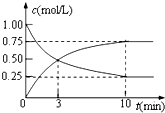 Ϊʵ�֡����ܼ��š��͡���̼���á���һ���������ν�CO2ת��Ϊ��������Դ�������ԭ������ C-12 H-1 O-16��
Ϊʵ�֡����ܼ��š��͡���̼���á���һ���������ν�CO2ת��Ϊ��������Դ�������ԭ������ C-12 H-1 O-16��