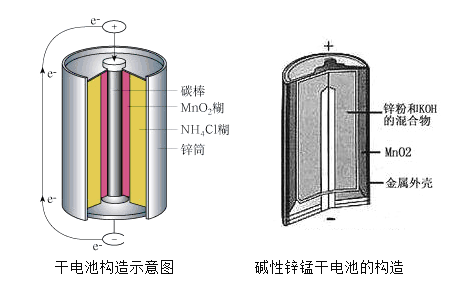
��Ŀ����
����Ŀ��ʵ�������ܶ�Ϊ1.19 g/mL����������Ϊ36.5%��Ũ��������500 mL 0.4 mol/L���ᡣ�ش��������⣺
(1)��Ũ������HCl�����ʵ���Ũ��Ϊ______��
(2)��������Ũ���������ˮ����500 mL 0.4 mol/L���ᡣ
������ȡ______mL����Ũ����������ơ�
�ڸ�����ʵ��������Ҫ����Ҫ������������Ͳ���ձ�����������_____________��
��������Ũ��������0.4 mol/L���ᣬ���ݵIJ�����_____________________��
(3)����500 mL 0.4 mol/L����ʱ,���в����лᵼ�½��ƫ�͵���____________(�����)��
a.����Ͳ��ȡŨ����ʱ������Ͳ�Ŀ̶�
b.����Ͳ��ȡŨ�����ϴ����Ͳ������ϴ��Һת������ƿ��
c.ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�ˮ���̶���
d.����������Һ������ƿת�Ƶ��Լ�ƿ��ʱ��������Һ�彦��
���𰸡�11.9 mol/L 16.8 ��ͷ�ιܺ�500 mL����ƿ �ز�����������ƿ��עˮ������̶���1��2 cm�������ý�ͷ�ιܣ��μ���Һ��İ�Һ����ʹ���̶������� c
��������
��1��Ũ��������ʵ���Ũ��Ϊ��![]() mol/L��
mol/L��
��2��������Ũ��������ΪV������V��11.9mol/L=500mL��0.4mol/L����V=16.8mL�����Ƹ���Һ��Ҫ�IJ�������Ϊ���ձ�����������500mL����ƿ����ͷ�ιܣ����ݵľ������Ϊ���ز�����������ƿ��עˮ������̶���1��2 cm�������ý�ͷ�ιܣ��μ���Һ��İ�Һ����ʹ���̶������С�
��3������a�У�����Ͳ��ȡŨ����ʱ������Ͳ�Ŀ̶ȣ���ȡ��Ũ����ƫ�࣬Ũ��ƫ����b�У�����Ͳ��ȡŨ�����ϴ����Ͳ������ϴ��Һת������ƿ�У����ʵ����ʵ������ӣ�Ũ��ƫ�ߣ�����c�У�ҡ�Ⱥ���Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߣ�ˮ�Ӷ࣬��Ũ��ƫС������d�У�����������Һ������ƿת�Ƶ��Լ�ƿ��ʱ��������Һ�彦����Ũ�Ȳ�Ӱ�졣

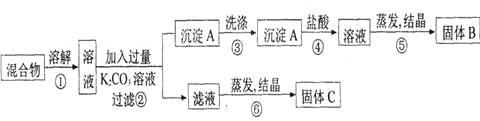
 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�����Ŀ����֪��Ӧ2CH3OH(g)![]() CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g)����ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£����ܱ������м���CH3OH����Ӧ���е�ijʱ�̲�ø���ֵ�Ũ�����£�
���� | CH3OH | CH3OCH3 | H2O |
Ũ��/mol��L��1 | 0.44 | 0.6 | 0.6 |
������������ȷ����(����)
A. ����CH3OH��Ũ�ȣ���ʹ����Ӱٷ������࣬��Ӧ���ʼӿ�
B. ��ʱ�������淴Ӧ���ʵĴ�С��v����v��
C. ƽ��ʱc(CH3OH)��0.04 mol��L��1
D. ������CH3OH����10 min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v(CH3OH)��1.6 mol��L��1��min��1
����Ŀ�����и��������У���������ͼ��ʾת����ϵ���ǣ���Ӧ������ȥ����ͷ��ʾһ��ת������ ��
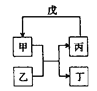
ѡ�� | �� | �� | �� | �� |
A | NH3 | Cl2 | N2 | H2 |
B | C | SiO2 | CO | CuO |
C | Al��OH��3 | NaOH | NaAlO2 | CO2 |
D | Br2 | FeI2 | FeBr3 | Cl2 |
A. AB. BC. CD. D