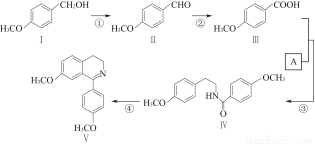
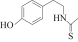
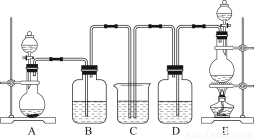
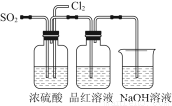
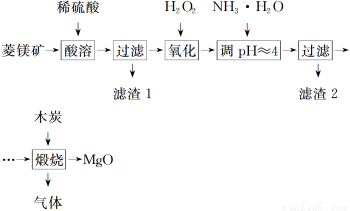
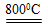��Ŀ����
ͭ��������������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�졣
��1��д��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
��2��Ϊ�˱��������ͽ�Լ��Դ��ͨ������H2O2��ϡ����Ļ����Һ�ܳ��Ͼ�ӡˢ��·���е�ͭ������ʵ��ͭ�Ļ������á�д���ܳ�ͭ�����ӷ���ʽ��________________________________________________________________________________________________________________________________________________��
��3����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O��Cu2S 6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
��4��ͭ�ڳ�ʪ�Ŀ������ܷ���������ʴ�����⣬ͭ�����Ҫ�ɷ�ΪCu2��OH��2CO3����ʽ̼��ͭ������д�����������и����ĵ缫��Ӧʽ��________________________________________________________________________________________________________________________________________________��
��5���о���ѧϰС��������ӵ��������ⶨij����CuSO4��5H2O����������I����Ӧ�����������ʣ��ĺ�����ȡa g�������100 mL��Һ��ÿ��ȡ25.00 mL���μ�KI��Һ���а�ɫ�⻯��������ɡ�д���÷�Ӧ�����ӷ���ʽ��___________________________�������μ�KI��Һ���������ٲ�������Һ�е�I2�����������Ʊ���Һ�ζ���������Ӧ�Ļ�ѧ����ʽΪI2��2Na2S2O3=2NaI��Na2S4O6��ƽ������c mol/L��Na2S2O3��ҺV mL����������CuSO4��5H2O����������Ϊ______________��
��1��3Cu��8HNO3��ϡ��=3Cu��NO3��2��2NO����4H2O
��2��Cu��H2O2��2H��=Cu2����2H2O
��3��Cu2O��Cu2S��0.3
��4��2Cu��4OH����CO2��4e��=Cu2��OH��2CO3��H2O
��5��2Cu2����4I��=2CuI����I2��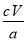 ��100%
��100%
����������2��H2O2�������������ܽ�Cu����ΪCu2������3���÷�Ӧ��Cu2S��Cu2O��Cu�Ļ��ϼ۾��ɣ�1�۽���ΪCu�е�0�ۣ�Cu2S��Cu2O��Ϊ����������Ԫ�ػ��ϼ��ɣ�2�����ߵ���4�ۣ�ת�Ƶ�����Ϊ6����������0.3 mol Cuʱת�Ƶ���Ϊ0.3 mol����4��Cu�ڸ���ʧȥ�����γ�Cu2����Cu2����������е�CO2��H2O �������ͭ�⡣��5�����ݵ����غ�ù�ϵʽ��Cu2����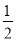 I2��Na2S2O3����������CuSO4��5H2O����������Ϊ
I2��Na2S2O3����������CuSO4��5H2O����������Ϊ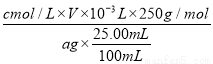 ��100%��
��100%��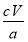 ��100%��
��100%��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�