��Ŀ����
20����4�ֺ�����Ԫ�صĻ�����A��B��C��D�����ܷ������з�Ӧ��A+NaOH��D+H2O�� ��B$\stackrel{����}{��}$A+H2O�� ��C+NaOH$\stackrel{����}{��}$B+NaCl
����D��Һ�е���������г������ɣ���������������ܽ�����C��
��1��д��B��C��D�Ļ�ѧʽ��BAl��OH��3��CAlCl3��DNaAlO2��
��2��д�����Ϣ١��۵����ӷ�Ӧ����ʽ��
��Al2O3+2OH-=2AlO2+H2O
��Al3++3OH-=Al��OH��3����
���� 4�ֺ�����Ԫ�صĻ�����A��B��C��D���ɢ���D��Һ�е���������г������ɣ���������������ܽ�����C������֪DΪƫ�����Σ�CΪAlCl3���ɷ�Ӧ��B$\stackrel{����}{��}$A+H2O����Ӧ��C+NaOH$\stackrel{����}{��}$B+NaCl����֪B��Al��OH��3��A��Al2O3���ɢ�A+NaOH��D+H2O����֪D��NaAlO2���Դ˽����⣮
��� �⣺��1��4�ֺ�����Ԫ�صĻ�����A��B��C��D���ɢ���D��Һ�е���������г������ɣ���������������ܽ�����C������֪DΪƫ�����Σ�CΪAlCl3���ɷ�Ӧ��B$\stackrel{����}{��}$A+H2O����Ӧ��C+NaOH$\stackrel{����}{��}$B+NaCl����֪B��Al��OH��3��A��Al2O3���ɢ�A+NaOH��D+H2O����֪D��NaAlO2��
�ʴ�Ϊ��Al��OH��3��AlCl3��NaAlO2��
��2����Ӧ�ٵ����ӷ���ʽΪ��Al2O3+2OH-=2AlO2+H2O��
��Ӧ�۵����ӷ�Ӧ����ʽΪ��Al3++3OH-=Al��OH��3����
�ʴ�Ϊ��Al2O3+2OH-=2AlO2+H2O��Al3++3OH-=Al��OH��3����
���� ���⿼��������ƶϣ��漰AlԪ�ػ������������ת������Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ������ÿ�ʱ�Ż���ҵϵ�д�
������ÿ�ʱ�Ż���ҵϵ�д�
�����Ŀ
18�������£�������������pH=1����Һ�в��ܴ���������ǣ�������
| A�� | Al3+��Na+��NO3-��Cl- | B�� | Fe3+��Na+��C1-��NO3- | ||
| C�� | K+��NH4+��Cl-��AlO2- | D�� | Cu2+��NH4+��SO42-��NO3- |
8���������ΪC4H9OH���ܷ�����������Ӧ�����ܵõ�ȩ���л��������У�������
| A�� | 4�� | B�� | 3�� | C�� | 2�� | D�� | 1�� |
12��ͬ��ͬѹ�£����и����Ȼ�ѧ����ʽ�У���H1����H2 ���ǣ�������
| A�� | C��s��+$\frac{1}{2}$O2��g���TCO��g������H1 C��s��+O2��g���TCO2��g������H2 | |
| B�� | $\frac{1}{2}$H2��g��+$\frac{1}{2}$Cl2��g���THCl��g������H1 H2��g��+Cl2��g���T2HCl��g������H2 | |
| C�� | 2H2��g��+O2��g���T2H2O��g������H1 2H2��g��+O2��g���T2H2O��l������H2 | |
| D�� | S��g��+O2��g���TSO2��g������H1 S��s��+O2��g���TSO2��g������H2 |
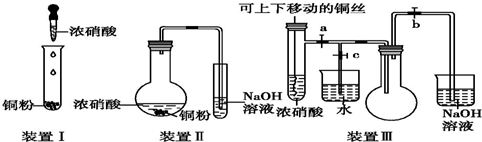
 ��
�� �������ȼҵ����Ҫ��Ʒ֮һ����һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����Cl2+H2O?HCl+HClO K=4.5��10-4
�������ȼҵ����Ҫ��Ʒ֮һ����һ�ֳ��õ���������������ԭ������ˮ��Ӧ�����˴����Cl2+H2O?HCl+HClO K=4.5��10-4 ��ͼװ���У�a��b���Ƕ��Ե缫��A��Bװ���е���Һ���������ģ�ͨ��һ��ʱ���Bװ����b��������Һ�ʺ�ɫ��
��ͼװ���У�a��b���Ƕ��Ե缫��A��Bװ���е���Һ���������ģ�ͨ��һ��ʱ���Bװ����b��������Һ�ʺ�ɫ��