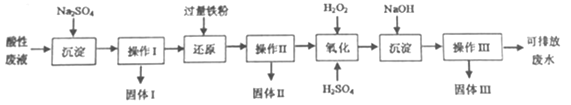
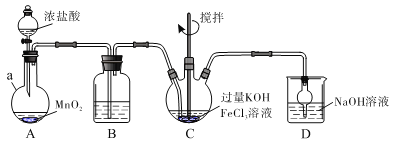
��Ŀ����
����Ŀ���ڵ����仯����Ļ��������У����йط�Ӧ�ķ�Ӧԭ�������о�������Ҫ���塣
��1��t��ʱ������N2��NH3��������Ӧ����Ϣ���±���ʾ��
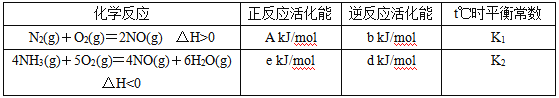
��д��t��ʱ������һ����������������������Ȼ�ѧ����ʽ��______________________��t��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ__________ (��K1��K2��ʾ)��
��2����ҵ�ϳɰ���ԭ��Ϊ��N2(g)��3H2(g)![]() 2NH3(g)��ͼ�ױ�ʾ��һ��������ܱ������з�Ӧʱ��H2�����ʵ���Ũ����ʱ��ı仯��ͼ�ұ�ʾ�������������������£���ʼͶ��H2��N2�����ʵ���֮��(��Ϊx)��ƽ��ʱNH3�����ʵ��������Ĺ�ϵ��
2NH3(g)��ͼ�ױ�ʾ��һ��������ܱ������з�Ӧʱ��H2�����ʵ���Ũ����ʱ��ı仯��ͼ�ұ�ʾ�������������������£���ʼͶ��H2��N2�����ʵ���֮��(��Ϊx)��ƽ��ʱNH3�����ʵ��������Ĺ�ϵ��
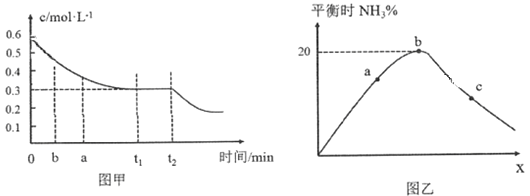
��ͼ����0��t1 min�ڣ�v(N2)��_____mol��L��1��min��1��b���v(H2)��_____a���v(H2)��(������������С��������������)��
��ͼ���У�b��ʱN2�����ʵ�������__________��
����֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��ƽ��________(���������ƶ����������ƶ����������ƶ���)��
���𰸡�4NH3(g)+6NO(g)=5N2(g)+6H2O(g) ��H=[(e-d)-5(A-b)] kJmol-1 ![]()
![]() molL-1min-1 ���� 20% ���ƶ�
molL-1min-1 ���� 20% ���ƶ�
��������
(1)���ݱ������ݣ���Ӧ����H=����Ӧ�Ļ��-�淴Ӧ�Ļ�ܼ������Ӧ�ٺ͢ڵ���H���ٸ��ݸ�˹���ɷ������
(2)�ٸ���ͼ��0��t1 min�ڣ���c(H2)=(0.6-0.3)molL-1=0.3molL-1������c (N2)=0.1molL-1���ݴ˼���v(N2)����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1���ݴ˷����жϣ�
�ڵ�H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1��ƽ��ʱ���������ʵ�������Ϊ20%����������ʽ�������㣻
�ۼ�֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L���������ʱ��ƽ�ⳣ��K�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L�������Qc������K�Ƚ��жϡ�
(1)���ݱ������ݣ���Ӧ����H=����Ӧ�Ļ��-�淴Ӧ�Ļ�ܣ���N2(g)+O2(g)=2NO(g) ��H=(A-b) kJmol-1����4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ��H=(e-d) kJmol-1�����ݸ�˹���ɣ�����-����5�õ�������һ����������������������Ȼ�ѧ����ʽ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g) ��H=[(e-d)-(A-b)��5]kJmol-1=[(e-d)-5(A-b)]kJmol-1��t��ʱ�÷�Ӧ��ƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H=[(e-d)-5(A-b)] kJmol-1��
���ʴ�Ϊ��4NH3(g)+6NO(g)=5N2(g)+6H2O(g)��H=[(e-d)-5(A-b)] kJmol-1��![]() ��
��
(2)��0��t1 min�ڣ���c(H2)=(0.6-0.3)molL-1=0.3molL-1������c (N2)=0.1molL-1����v(N2)=![]() =
=![]() =
=![]() molL-1min-1����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1�������������䣬��ƽ��ʱ������Ũ��С��b����b���v(H2)����a���v(H2)�����ʴ�Ϊ��
molL-1min-1����H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1����a��xС��3��1�������������䣬��ƽ��ʱ������Ũ��С��b����b���v(H2)����a���v(H2)�����ʴ�Ϊ��![]() molL-1min-1�����ڣ�
molL-1min-1�����ڣ�
�ڵ�H2��N2����ʼ����ȷ��Ϸ���ʽ�л�ѧ������֮��ʱ���ﵽƽ��ʱ��������������ͼ���У�b��ʱx=3��1��ƽ��ʱ���������ʵ�������Ϊ20%������ʼʱH2��N2�����ʵ����ֱ�Ϊ3mol��1mol�����ɵİ���Ϊ2xmol
N2(g)��3H2(g) 2NH3(g)
��ʼ(mol) 1 3 0
��Ӧ(mol) x 3x 2x
ƽ��(mol)1-x 3-3x 2x
��![]() ��100%=20%�����x=
��100%=20%�����x=![]() ��b��ʱN2�����ʵ�������=
��b��ʱN2�����ʵ�������=![]() =20%���ʴ�Ϊ��20%��
=20%���ʴ�Ϊ��20%��
�ۼ�֪ij�¶��¸÷�Ӧ��ƽ��ʱ�����ʾ�Ϊ1 mol���������Ϊ1L����ʱ��ƽ�ⳣ��K=![]() =1�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L��Qc=
=1�������¶Ⱥ�ѹǿ���䣬�ֳ���3 mol N2��������������2L��Qc=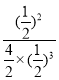 =1=K��Ϊƽ��״̬��ƽ�ⲻ�ƶ����ʴ�Ϊ�����ƶ���
=1=K��Ϊƽ��״̬��ƽ�ⲻ�ƶ����ʴ�Ϊ�����ƶ���