��Ŀ����
����Ŀ������������Ԫ�� A��B��C��D ��ԭ������������������ C Ϊ����Ԫ�أ�A��C ��������������ȣ�C��D ��Ԫ��ԭ�ӵ�������֮��Ϊ A��B ��Ԫ��������֮�͵� 3 ����
(1)д�� A��B��C��D ��Ԫ�ط���:A_________,B_________,C_________,D_________ ��
(2)A��B��C��D ��ԭ�Ӱ뾶��С˳��Ϊ _________��
(3)A �� C �γɻ����� CA �ĵ���ʽΪ_________��
(4) �õ���ʽ��ʾC��D�γɻ����� C2D �� �� �� : _________��
���𰸡�H O Na S r(Na)��r(S)��r(O)��r(H) Na+[:H] ![]()
��������
����������Ԫ��A��B��C��D��ԭ������������������CΪ����Ԫ�أ�C��������������A��ȣ���A��CΪͬ��Ԫ�أ���A��C����Ϊ����ƻ����������C��D��Ԫ��ԭ�ӵ�������֮��ΪA��B��Ԫ��������֮�͵�3�������A��CΪ���������B��ԭ������Ϊx��D��ԭ������Ϊy������3��(5+x)=13+y��x��5����D��ԭ����������18�����������⣬����AΪ�⣬CΪ�ƣ�����3��(1+x)=13+y����DΪ�ǽ�����D��ԭ�����������ƿ�֪��BΪ��Ԫ�أ�DΪ��Ԫ�أ��ݴ˴��⡣
(1)��������ķ�����֪��AΪH��BΪO��CΪNa��DΪS��
(2)���ݵ��Ӳ���Խ�࣬�뾶Խ���Ӳ�����ͬ���˵����Խ�࣬�뾶ԽС��֪��A��B��C��D��ԭ�Ӱ뾶��С˳��Ϊr(Na)��r(S)��r(O)��r(H)��
(3)A��C�γɻ�����ΪNaH�����ĵ���ʽΪNa+[:H]��
(4) CΪNa��DΪS��C��D�γɻ�����ΪNa2S���õ���ʽ��ʾNa2S���γɹ���Ϊ![]() ��
��

 ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д� �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�����Ŀ��I�����������н���ԭ����Ҫ�����ֳ����Ķѻ���ʽ�����������ѻ������������ѻ��������ѻ���
(1)����ͭ��������________(����ĸ����)�ѻ���ʽ��
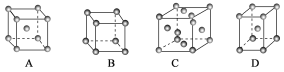
(2)�ྻ��(�����ںϳɰ���Ӧ�Ĵ���)�ı����ϴ��ڵ�ԭ�ӣ���ͼΪ��ԭ�������ľ����ϵĵ��㸽�žֲ�ʾ��ͼ(ͼ��С��ɫ�������ԭ�ӣ���ɫ�������ԭ��)������ͼʾ״���£�������������N/Feԭ������ֵ�����ֵΪ________________��
II����֪A��B��C��D��E����Ԫ�ض���Ԫ�����ڱ���ǰ20��Ԫ�أ�ԭ��������������E����Χ�����Ų�ʽΪ4s2��A��B��C��D����Ԫ����Ԫ�����ڱ��е����λ�����±���ʾ��
���� | A | ||||||
B | C | D |
����������Ϣ���ش��������⣺
(1)A��B�������У��뾶��С����________(�����ӷ���)��
(2)A��D�ֱ���B�γɵĻ������У�________�ľ����ܴ�(�ѧʽ)��
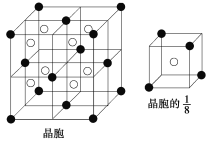
(3)A��E��������ӻ�����侧��(�������ھ����о��д����Ե���С�ظ���Ԫ)�ṹ��ͼ��ʾ(��������������ʾ��λ�ڸ�������Ķ�������ģ�����������������ʾ����λ��С����������)���û�����ĵ���ʽ��____________��A��E������ľ���1/8�����Ϊ2.0��10��23 cm3����A��E��ɵ����ӻ�������ܶȣ�����ʽ������(�������һλС��)��__________________________________________��
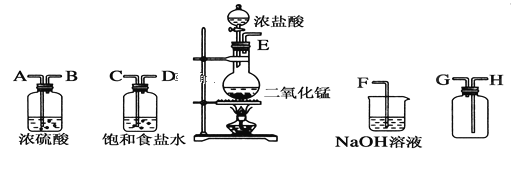
����Ŀ���ʰ�������[(NH2CH2COO)2Fe]��һ�ֲ���ǿ������ijѧϰС������FeCO3��ʰ���(NH2CH2COOH)�Ʊ��ʰ���������ʵ��װ������ͼ��ʾ(�гֺͼ���������ʡ��)��
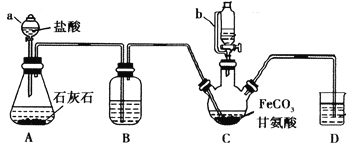
�й������������±���
�ʰ��� | ������ | �ʰ������� |
������ˮ�������Ҵ� | ������ˮ���Ҵ� | ������ˮ���������Ҵ� |
���Ի����� | ǿ���ԡ�ǿ��ԭ�� |
ʵ����̣�
I���ϳɣ�װ��C��ʢ��0.1mol FeCO3��200mL1.0mol��L-1�ʰ�����Һ�����������ᡣʵ��ʱ���ȴ�����a�Ļ�������װ��c�п����ž����Ȳ����Ͻ��裬��ͨ������b��C�м�����������������Һ����p�ȵ�6���ң�ʹ��Ӧ���ַ�Ӧ��
�������룺��Ӧ�������ˣ�����Һ��������Ũ����������ˮ�Ҵ������ˡ�ϴ�Ӳ����
�ش��������⣺
��1������a��������________����a��ȣ�����b���ŵ���_____________________________��
��2��װ��B��ʢ�е��Լ���____________��ʵ�������װ��D�ĵ���һֱû��Һ���µı�Ҫ����___________________________________________��
��3���ϳɹ��̼���������������Ǵٽ�FeCO3�ܽ��________________________ ��
��4����������������Һ����pH������6���ʰ������������½���ԭ��������ӷ���ʽ��ʾΪ________��
��5������II�м�����ˮ�Ҵ���Ŀ����_______________________��
��6�������Ʒ���Ƿ���Fe3+���Լ�������_________ ��
��7����ʵ���Ƶ�15.3g�ʰ���������M=204g/mol�������������_____����