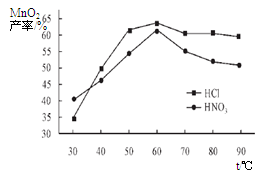
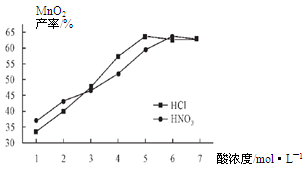
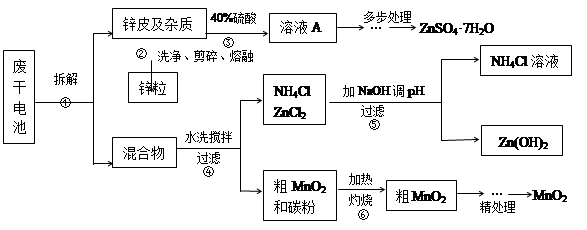��Ŀ����
��������Ҫ�ɷ�ΪFeO��Cr2O3������������Al2O3��һ���������Cr2O3��������ԼΪ40�����ɸ������Ʊ��ظ���صķ������£�

��֪��
��4FeO��Cr2O3��8Na2CO3��7O2 8Na2CrO4��2 Fe2O3��8CO2����
8Na2CrO4��2 Fe2O3��8CO2����
��Na2CO3��Al2O3 2NaAlO2��CO2����
2NaAlO2��CO2����
�� Cr2O72����H2O 2CrO42����2H+
2CrO42����2H+
��������ش��������⣺
��1������IΪ ������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ�� ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH<5����Ŀ���� ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4������Y����Ҫ����������������д��������Һ��pH=7~8ʱ�����������������ӷ���ʽ ��

��֪��
��4FeO��Cr2O3��8Na2CO3��7O2
 8Na2CrO4��2 Fe2O3��8CO2����
8Na2CrO4��2 Fe2O3��8CO2������Na2CO3��Al2O3
 2NaAlO2��CO2����
2NaAlO2��CO2������ Cr2O72����H2O
 2CrO42����2H+
2CrO42����2H+��������ش��������⣺
��1������IΪ ������X����Ҫ���� ����д��ѧʽ����Ҫ����ữ��������Һ��pH�Ƿ����4.5��Ӧ��ʹ�� ����д�������Լ����ƣ���
��2���ữ�����ô��������ҺpH<5����Ŀ���� ��
��3���������жಽ��ɣ����K2Cr2O7����IJ��������ǣ�����KCl���塢����Ũ���� �����ˡ� �����
��4������Y����Ҫ����������������д��������Һ��pH=7~8ʱ�����������������ӷ���ʽ ��
��1�����ˣ�2�֣��� Fe2O3��2�֣��� pH�ƻ���pH��ֽ�Ͳ�������2�֣���
��2������H+Ũ�ȿ�ʹ��ѧƽ��Cr2O72����H2O
 2CrO42����2H+�����ƶ���ʹCrO42��ת��ΪCr2O72���������ش�����֣���2�֣���
2CrO42����2H+�����ƶ���ʹCrO42��ת��ΪCr2O72���������ش�����֣���2�֣�����3����ȴ�ᾧ��2�֣��� ϴ�ӣ�2�֣���
��4��CH3COOH��AlO2����H2O = Al(OH)3����CH3COO�� ��2�֣�
����������������պ�IJ�����Na2CrO4��Fe2O3��NaAlO2����ˮ��ȡ��������ˮ����Fe2O3��Ҳ����X�����˷��룬�Ӵ����PH��7~8��ʹAlO2-ת��ΪAl(OH)3���������˷��룬Ҳ����Y��������PH<5��ʹCrO42��ת��ΪCr2O72������KCl�����ᾧ�ȵ��ظ����

��ϰ��ϵ�д�
�����Ŀ