��Ŀ����
���������(NH4)2Fe(SO4)2��6H2O��һ��dz��ɫ���壬��ˮ�е��ܽ�Ƚ�С���������Ҵ���ijʵ��С�����ö�п��Ƭ���Ʊ���������淋Ĺ������£�

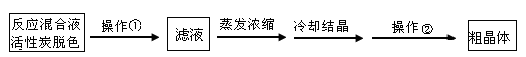
��1������������Ϊ�˳�ȥп�Ʋ㣬�ж�п�Ʋ��ѱ���ȥ�������� ��
��2��A���ʿ����� ��ѡ����ţ���
a��CuCl2 b��CuO c��Cu(NO3)2 d��CuSO4
��������A���ʵ�Ŀ���� ��
��3�������������Ϊ ��
��4���������ɶ����ü��Ⱥ�ɾ����ԭ���� ��
��5����ҵ�ϳ���K2Cr2O7��Һ�ⶨ��������淋Ĵ��ȣ���Ӧ��Cr2O72������ԭ��Cr3+��
д�������������Һ������K2Cr2O7��Һ��Ӧ�����ӷ���ʽ ��

��1������������Ϊ�˳�ȥп�Ʋ㣬�ж�п�Ʋ��ѱ���ȥ�������� ��
��2��A���ʿ����� ��ѡ����ţ���
a��CuCl2 b��CuO c��Cu(NO3)2 d��CuSO4
��������A���ʵ�Ŀ���� ��
��3�������������Ϊ ��
��4���������ɶ����ü��Ⱥ�ɾ����ԭ���� ��
��5����ҵ�ϳ���K2Cr2O7��Һ�ⶨ��������淋Ĵ��ȣ���Ӧ��Cr2O72������ԭ��Cr3+��
д�������������Һ������K2Cr2O7��Һ��Ӧ�����ӷ���ʽ ��
16����12�֣�
��1����Ӧ����ͻȻ��С������Ƭ�������ɵ�����ͻȻ���٣���˼��������֣���2�֣�
��2��b��d��2�֣����1����1�֣����2����2�֣����������֣�
�ӿ�����ϡ����ķ�Ӧ���ʣ�2�֣�
��3�����ˣ�2�֣�
��4��������ȹ����о������ȷֽ�ʧȥ�ᾧˮ����������2�֣�
��5��6Fe2+��Cr2O72����14H+��2Cr3+��6Fe3+��7H2O��2�֣�
��1����Ӧ����ͻȻ��С������Ƭ�������ɵ�����ͻȻ���٣���˼��������֣���2�֣�
��2��b��d��2�֣����1����1�֣����2����2�֣����������֣�
�ӿ�����ϡ����ķ�Ӧ���ʣ�2�֣�
��3�����ˣ�2�֣�
��4��������ȹ����о������ȷֽ�ʧȥ�ᾧˮ����������2�֣�
��5��6Fe2+��Cr2O72����14H+��2Cr3+��6Fe3+��7H2O��2�֣�
���������
��1����п��Ƭ���������п����γ�Zn-HCl-Feԭ��أ������ܼӿ�H+�ķ�Ӧ���ʣ���������������ͻȻ���١�
��2�������A������Ҫ��Ϊ������Ӧ�ӿ����ʣ�ͨ������Fe-H2SO4-Cuԭ�����ʵ�֣�������Һ��Ҫ����FeSO4������a��CuCl2����ѡ�����ջ���FeCl2���ʣ�c��Cu(NO3)2 �����NO3-���ӣ���������ж������������һ��������Cu(NO3)2��ֻ��b��CuO ��d��CuSO4��������������ܼӿ췴Ӧ���ʡ�
��3�������������Ϊ���ˣ���ȥδ��Ӧ�������ͭ��
��4������Ŀ������(NH4)2Fe(SO4)2��6H2O�еļ��нᾧˮ��ͬʱ��Ϊ+2 �ۣ������ü��Ⱥ�ɿ��ܻ�ʧȥ�ᾧˮ�ͼӿ�+2 �۵�������
��5������������ԭ���ɣ�һ����Ӧ����Ԫ�ػ������ߣ���Ȼ�н��͵ģ���Ŀ�Ѿ�������Cr2O72������ԭ��Cr3+Ԫ�ػ��ϼ��ǽ��͵ģ������������Һ��ֻ��Fe2+���ϼ����ߵ�+3�ۣ��ȸ��ݵ�ʧ�������д��6Fe2+��Cr2O72����2Cr3+��6Fe3+���ٸ�����Һ������ͨ������غ���ɷ���ʽȱ����ƽ�����H+���ڱ�H2O��

��ϰ��ϵ�д�
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
�����Ŀ

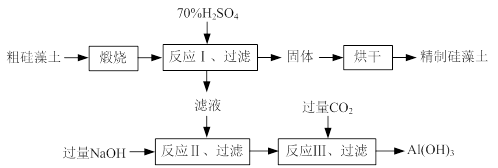
 8Na2CrO4��2 Fe2O3��8CO2����
8Na2CrO4��2 Fe2O3��8CO2���� 2CrO42����2H+
2CrO42����2H+
 Na2S2O3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ����������ͼ��ʾ��
Na2S2O3����������Һ����������ΪNa2S2O3?5H2O��Na2S2O3��5H2O��40��45���ۻ���48��ֽ⣻Na2S2O3������ˮ���������Ҵ�����ˮ���й����ʵ��ܽ����������ͼ��ʾ��

 ��100%�����м��㡣�ɴ˷�������5�еζ���Ӧ�����ӷ���ʽΪ ��
��100%�����м��㡣�ɴ˷�������5�еζ���Ӧ�����ӷ���ʽΪ ��