��Ŀ����
����Ŀ����������������ϵʾ��ͼ����������˵����ȷ����( )
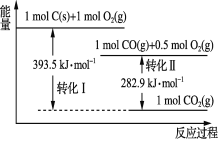
A.1 mol C(g)��1 mol O2(g)������֮��Ϊ393.5 kJ
B.��Ӧ2CO(g)��O2(g)=2CO2(g)�У���Ӧ����ܼ���С����������ܼ���
C.C��CO���Ȼ�ѧ����ʽΪ2C(s)��O2(g)=2CO(g)����H����110.6 kJ��mol��1
D.��ֵ��ָһ�������µ�λ������������ȫȼ�����ų�����������CO����ֵΪ282.9 kJ��g��1
���𰸡�B
��������
A����ͼ��֪��1molC��s����1molO2��g��������֮�ʹ���393.5kJ����1molC��g����1molO2��g��������֮��Զ����393.5kJ����A����
B����ͼ��֪��1molCO��g����0.5mol��O2��g������1molCO2��g���ų�282.9kJ�����������Է�Ӧ2CO��g��+O2��g���T2CO2��g���У���Ӧ������������������������������Ӧ����ܼ���С����������ܼ��ܣ���B��ȷ��
C����ͼ��֪��1molC��s����0.5molO2��g��ת��Ϊ1mol��CO��g�����ų�����Ϊ��393.5 kJ -282.9 kJ =110.6kJ������2C��s��+O2��g���T2CO��g����H=-221.2kJ/mol����C����
D����ֵָ��һ��������ÿ��������ȫȼ�����ų���������ȼ�ղ����ڸ���������һ�ֽ�Ϊ�ȶ���״̬����CO����ֵΪ![]() =10.1kJ/g����D����
=10.1kJ/g����D����
��ѡB��

����Ŀ���о�����������ķ�Ӧ��ʵ�����¡�
ʵ�� (20C) | ���� |
�� | ��ɫ����(�����������ɫ)����Һ��Ϊ��ɫ |
�� | 6mL��ɫ����(�����ΪH2)����Һ������ɫ |
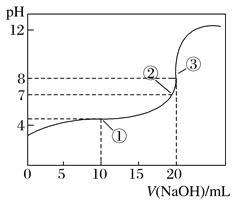
�����в�������ɫ������______��
������������
�ټ�ͬѧ��Ϊ��Ũ�ȵ�������H+�������Դ���NO3��������NO3��û�з�����Ӧ����ͬѧ����������֤���˼�˵������ȷ����ʵ��֤����______��
![]() ��ͬѧͨ���������Ʋ��NO3��Ҳ�ܱ���ԭ��������______��������ͨ��ʵ��֤ʵ����Һ�к���NH4+����ʵ�������______��
��ͬѧͨ���������Ʋ��NO3��Ҳ�ܱ���ԭ��������______��������ͨ��ʵ��֤ʵ����Һ�к���NH4+����ʵ�������______��
![]() ��ȫ����NO3������ԭΪ
��ȫ����NO3������ԭΪ![]() �Ĺ��̣�NO3�� + ______e�� + ______= NH4+ + ______H2O
�Ĺ��̣�NO3�� + ______e�� + ______= NH4+ + ______H2O
���о�Ӱ�����H2������
ʵ�� | ���� |
�� | 3.4m L����(�����ΪH2������Һ�Դ���ɫ |
�� | 2.6m L����(�����ΪH2������ɫ��Һ |
�� | ��ɫ����(�����������ɫ)������ɫ��Һ |
���ϣ�Fe[(NO)]2+����Һ�г���ɫ�� �ܶԱ��������������¶Ȳ�ͬʱ�ռ��������������ͬ��ԭ����______�� ��������ɫ�����ǻ�����壬��һ�����������������ɫ�������______�� �ȸ���ʵ�飬���������ᷴӦʱ��Ӱ������Ļ�ԭ���ﲻͬ��������______��