��Ŀ����
����Ŀ�����±���������������ѡ���ʵ�������������ֵ����(ÿ������ֻ��ѡ��һ��)���ö��Ե缫��ÿ�ֵ������Һ���е�⡣�ش��������⣺
������ | H+��Na+��Ag+ |
������ | Cl-��SO42-��NO3- |
��1���������ų������������ų��������ҵ�����Һ��pH��С������ѡ�õĵ���ʵĻ�ѧʽ��___________�������ĵ缫��ӦʽΪ___________��
��2�����������������������ų�����������ѡ�õĵ���ʵĻ�ѧʽ��___________�������ĵ缫��ӦʽΪ_______________________________________________________________��
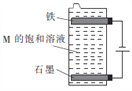
��3������ͼ��ʾװ�õ������ֵ����M�ı�����Һ��д���õ����з�����Ӧ���ܻ�ѧ����ʽ��____________________________��
���𰸡� H2SO4 2H++2e-=H2�� AgNO3 4OH--4e-=O2��+2H2O(��2H2O--4e-=O2��+4H+) NaCl+H2O![]() NaClO+H2��
NaClO+H2��
��������������������⿼���ö��Ե缫���������Һ�Ĺ��ɣ��缫��Ӧʽ�͵���ܷ���ʽ����д������AgCl������ˮ��Ag2SO4����ˮ��Ag+ֻ����NO3-�γɵ������Һ��
��1�������ӷŵ�˳��ΪAg+![]() H+
H+![]() Na+�������ӵķŵ�˳��ΪCl-
Na+�������ӵķŵ�˳��ΪCl-![]() OH-
OH-![]() ����������������ų�H2�������ų�O2�������ϵ��ˮ���������Һ������ΪAgNO3��Һ���������Һ�в�������Cl-������ÿ������ֻ��ѡ��һ�Σ��������Һ����ΪH2SO4��Na2SO4��������Һ��pH��С�ĵ���ʵĻ�ѧʽΪH2SO4�������ĵ缫��ӦʽΪ2H++2e-=H2����
����������������ų�H2�������ų�O2�������ϵ��ˮ���������Һ������ΪAgNO3��Һ���������Һ�в�������Cl-������ÿ������ֻ��ѡ��һ�Σ��������Һ����ΪH2SO4��Na2SO4��������Һ��pH��С�ĵ���ʵĻ�ѧʽΪH2SO4�������ĵ缫��ӦʽΪ2H++2e-=H2����
��2�����������������������ų�O2����ѡ�����Ϊ�����ý����ĺ������Σ�����ѡ����ʵĻ�ѧʽΪAgNO3������ΪOH-�ŵ磬�����缫��ӦʽΪ4OH--4e-=O2��+2H2O��
��3������ÿ������ֻ��ѡ��һ�Σ�������ֵ������ҺΪNaCl��Һ���ڵ�����FeΪ�������缫��ӦʽΪ2H2O+2e-=H2��+2OH-��ʯīΪ�����������缫��ӦʽΪ2Cl--2e-=Cl2������ⷴӦΪ2NaCl+2H2O![]() 2NaOH+H2��+Cl2�����������ɵ�Cl2���������ɵ�NaOH������ӦCl2+2NaOH=NaCl+NaClO+H2O��������Ӧ��ӵõ����з�����Ӧ���ܻ�ѧ����ʽΪNaCl+H2O
2NaOH+H2��+Cl2�����������ɵ�Cl2���������ɵ�NaOH������ӦCl2+2NaOH=NaCl+NaClO+H2O��������Ӧ��ӵõ����з�����Ӧ���ܻ�ѧ����ʽΪNaCl+H2O![]() NaClO+H2����
NaClO+H2����

 �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д� ����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�
����ͼ����ּ��������ҵ֣�ݴ�ѧ������ϵ�д�