��Ŀ����
����Ŀ��ijУ�С���ͬѧ������ϩ����ԭ��������ķ�������ȡ������
��1������ͬѧ���Ҵ���ˮ�ķ�Ӧ����ȡ��������ϩ���壬�䷴Ӧԭ�����Ʊ�װ�����£�
����Ӧ:C2H5OH![]() CH2=CH2��+H2O
CH2=CH2��+H2O
����Ӧ:C2H5OH+2H2SO4��Ũ) ![]() C+2SO2��+5H2O;
C+2SO2��+5H2O;
C+H2SO4��Ũ) ![]() CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
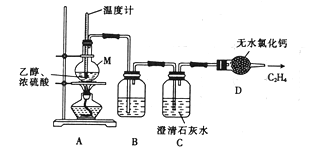
�� ����M��������_______________��
�� װ��B ��ʢ�ŵ��Լ�������____________��
�� װ��C ��������______________
��2������ͬѧ�ü���ͬѧ�Ƶõ���ϩ���������װ�ý�����ϩ����ԭ���������ʵ�顣
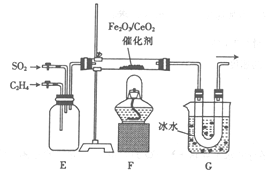
�� װ��E��������___________��װ��G��������_____________��
�� ����������������̼���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ_________��
�� ������װ�ü���G���ݳ������庬����������CO2��
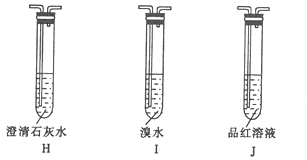
װ����ȷ������˳��ΪG![]() _________��֤��G���ݳ������庬��CO2������Ϊ_______��
_________��֤��G���ݳ������庬��CO2������Ϊ_______��
���𰸡� Բ����ƿ NaOH��Һ�������������𰸣� ����CO2��SO2�Ƿ���� ���C2H4��SO2���� �����ɵ���������ȴΪ���� C2H4+3SO2 ![]() 3S+2CO2+2H2O I
3S+2CO2+2H2O I![]() J
J![]() H Ʒ����Һ����ɫ������ʯ��ˮ�����
H Ʒ����Һ����ɫ������ʯ��ˮ�����
��������(1���� ����M��������Բ����ƿ��
�� ����ϩ�л���SO2��CO2��װ��B ��Ӧʢ��NaOH��Һ��ȥ���е������������壻
�� SO2��CO2����ʹ����ʯ��ˮ����ǣ� װ��C�������Ǽ���CO2��SO2�Ƿ������
(2���� װ��E��ʹC2H4��SO2������ȫ��ϲ���ͨ�����ƻ�������������ı�����װ��G���ñ�ˮ����オ�£�ʹ���ɵ���������ȴΪ���壻
�� ��ϩ��SO2��Ӧ����������̼�����ݵ���ת���غ㼰ԭ���غ㣬�÷�Ӧ�Ļ�ѧ����ʽΪC2H4+3SO2 ![]() 3S+2CO2+2H2O��
3S+2CO2+2H2O��
������������CO2�п��ܻ���δ��Ӧ��SO2����Ҫͨ����ˮ��ȥSO2��������Ʒ����Һ����SO2�Ƿ�ȫ����ȥ����װ����ȷ������˳��ΪG![]() I
I![]() J
J![]() H��������Ʒ����Һ����ɫ������ʯ��ˮ�������֤��G���ݳ������庬��CO2��
H��������Ʒ����Һ����ɫ������ʯ��ˮ�������֤��G���ݳ������庬��CO2��

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�