��Ŀ����
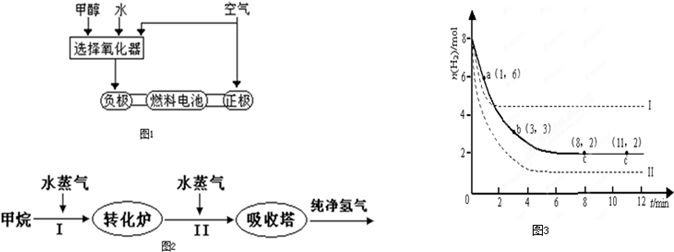
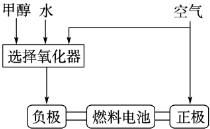
�¹�����˹��˾�ɹ����������ü״�����������ȼ�ϵ�ع��գ���ԭ����ͼ��ʾ����۲��ͼ�ش�

(1)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g)����H= + 49.0 kJ��mol-1
��2CH3OH(g)+O2(g)=2CO2(g)+4H2(g)����H=��385.8kJ��mol-1
����˵����ȷ����_____________
A. ��Ӧ���з�Ӧ��������������������������
B. ��Ӧ���в�CH3OH(g)��H2O(g)�еĻ�ѧ���������������γ�CO2(g)��3H2(g)�еĻ�ѧ�����ͷŵ�����
C. CH3OH������ȼ����Ϊ����192.9 kJ��mol-1
D. ���ݢ���֪��Ӧ��2CH3OH(l)+ O2(g)=2CO2(g)+4H2(g)�ġ�H����385.8kJ��mol-1
��2�������ѧ���������ɫ���ɡ����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״�������2.2kg CO2������H2ǡ����ȫ��Ӧ��������̬��ˮ�ͼ״����ɷų�2473.5 kJ����������д���ϳ����з�����Ӧ���Ȼ�ѧ����ʽ__________________��
��3���ɸ��ʼDZ����Թ���ļ״�ȼ�ϵ���Ѿ���������ṹʾ��ͼ��ͼ���״��ڴ����������ṩ���� (H+)�͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ�缫����������Ӧ������ܷ�ӦΪ��2CH3OH��3O2=2CO2��4H2O��b��ͨ���������____________��������ӦʽΪ��_____________________��
��CH3OH(g)+H2O(g)=CO2(g)+3H2(g)����H= + 49.0 kJ��mol-1
��2CH3OH(g)+O2(g)=2CO2(g)+4H2(g)����H=��385.8kJ��mol-1
����˵����ȷ����_____________
A. ��Ӧ���з�Ӧ��������������������������
B. ��Ӧ���в�CH3OH(g)��H2O(g)�еĻ�ѧ���������������γ�CO2(g)��3H2(g)�еĻ�ѧ�����ͷŵ�����
C. CH3OH������ȼ����Ϊ����192.9 kJ��mol-1
D. ���ݢ���֪��Ӧ��2CH3OH(l)+ O2(g)=2CO2(g)+4H2(g)�ġ�H����385.8kJ��mol-1
��2�������ѧ���������ɫ���ɡ����룺�ѿ�������̼�����Һ��Ȼ���ٰ�CO2����Һ����ȡ����������ѧ��Ӧ��ʹ�����е�CO2ת��Ϊ������ȼ�ϼ״�������2.2kg CO2������H2ǡ����ȫ��Ӧ��������̬��ˮ�ͼ״����ɷų�2473.5 kJ����������д���ϳ����з�����Ӧ���Ȼ�ѧ����ʽ__________________��
��3���ɸ��ʼDZ����Թ���ļ״�ȼ�ϵ���Ѿ���������ṹʾ��ͼ��ͼ���״��ڴ����������ṩ���� (H+)�͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ�缫����������Ӧ������ܷ�ӦΪ��2CH3OH��3O2=2CO2��4H2O��b��ͨ���������____________��������ӦʽΪ��_____________________��

��1��BCD
��2��CO2��g��+ 3H2��g��= H2O��g��+CH3OH��g�� ��H=-49.47kJ/mol
��3�������������CH3OH��H2O��6e-=CO2��6H+
��2��CO2��g��+ 3H2��g��= H2O��g��+CH3OH��g�� ��H=-49.47kJ/mol
��3�������������CH3OH��H2O��6e-=CO2��6H+

��ϰ��ϵ�д�
�����Ŀ
 �¹�����˹��˾�ɹ����������ü״�����������ȼ�ϵ�ع��գ���ԭ����ͼ1��ʾ����۲��ͼ�ش�
�¹�����˹��˾�ɹ����������ü״�����������ȼ�ϵ�ع��գ���ԭ����ͼ1��ʾ����۲��ͼ�ش�