��Ŀ����
3�� ������ͼװ�����ⶨijԭ��ع���ʱ��ij��ʱ����ͨ�����ߵĵ��ӵ����ʵ�������Ͳ�Ĺ��Ϊ1000mL����ѡ��ĵ缫�����д�ͭƬ�ʹ�пƬ����ش��������⣺
������ͼװ�����ⶨijԭ��ع���ʱ��ij��ʱ����ͨ�����ߵĵ��ӵ����ʵ�������Ͳ�Ĺ��Ϊ1000mL����ѡ��ĵ缫�����д�ͭƬ�ʹ�пƬ����ش��������⣺��1��b�缫����Ϊͭ����缫��ӦʽΪ2H++2e-�TH2����
��2������Ͳ���ռ���672mL����״���£�����ʱ��ͨ�����ߵĵ��ӵ����ʵ���Ϊ0.06mol����ʱa�缫������С������ӡ����١���1.95g��
��3�������a��b���缫�ĵ缫���϶Ե���U�ι��н����ֵ������������ҹ�½����Ҷ���ҹ������
���� ��1����ͭƬ�ʹ�пƬ��ϡ�������ԭ��أ���ͼ��֪b�缫�����������ɣ���bΪͭ��Ϊ������aΪп��Ϊ������b�������ӵõ�������������
��2�����ݵ缫����ʽ�����������ʵ���������ӵ����ʵ�����a��ΪZn������ʧ���ӣ����ݵ����غ����Zn��������
��3�������a��b���缫�ĵ缫���϶Ե������ұ�Ϊпʧ���������������ΪͭΪ�����������ӵõ�������������
��� �⣺��1����ͭƬ�ʹ�пƬ��ϡ�������ԭ��أ���ͼ��֪b�缫�����������ɣ���bΪͭ��Ϊ������aΪп��Ϊ������b�������ӵõ���������������缫��ӦʽΪ��2H++2e-�TH2����
�ʴ�Ϊ��ͭ��2H++2e-�TH2����
��2������Ͳ���ռ���672mL����״���£����壬��n��H2��=$\frac{V}{Vm}$=$\frac{0.672L}{22.4L/mol}$=0.03mol����֪b�ϵĵ缫��ӦʽΪ��2H++2e-�TH2������ͨ�����ߵĵ��ӵ����ʵ���Ϊ0.06mol��a�缫�ϵķ�ӦΪ��Zn-2e-�TZn2+�����ܽ��Zn�����ʵ���Ϊ0.03mol�����С��Zn������Ϊ65g/mol��0.03mol=1.95g��
�ʴ�Ϊ��0.06����С��1.95��
��3�������a��b���缫�ĵ缫���϶Ե������ұ�Ϊпʧ���������������ΪͭΪ�����������ӵõ�����������������������������ɣ�����U�ι��������ҹ�½����Ҷ���ҹ������
�ʴ�Ϊ�������ҹ�½����Ҷ���ҹ������
���� ���⿼����ԭ��صĹ���ԭ��֪ʶ��Ϊ��Ƶ���㣬����ѧ���ķ��������������Ŀ��飬��Ŀ��Ҫ�������������жϡ��缫����ʽ����д�������غ��ڼ����е�Ӧ�õȣ�ע��֪ʶ�Ļ����ǽ���ؼ����Ѷ��еȣ�

| A�� | �Ҵ�����������ȩ | B�� | �Ҵ���Ũ���Ṳ������ϩ | ||
| C�� | ����ϩ��һ���������ƾ۱���ϩ | D�� | ���������Ҵ� |
| A�� | Al��OH��3��Al2O3 | B�� | Al2O3��Al��OH��3 | C�� | Al��Al��OH��3 | D�� | SiO2��H2SiO3 |
| Ԫ�� | �� | �� | �� | �� | �� |
| ���ϼ� | -4 | +1 | -4 | -2 | -1 |
| A�� | �ҵij��������������� | |
| B�� | ��̬�⻯���ȶ��ԣ������� | |
| C�� | ������������������⻯���ˮ��Һ��Ӧ | |
| D�� | ԭ�Ӱ뾶��С���ף��� |
| ѡ�� | ʵ����� | ���� | ���� |
| A | ����Fe��OH��2¶���ڿ�����һ��ʱ�� | ��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ | ˵��Fe��OH��2�ױ�������Fe��OH��3 |
| B | �����½�FeƬ����Ũ������ | �����Ա仯 | Fe��Ũ�����Ӧ |
| C | ��һС��Na����ҽ�þƾ��� | �������� | Naֻ�û������ǻ��ϵ��� |
| D | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | 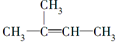 +Br2��CCl4���� +Br2��CCl4���� | |
| B�� | CH2=CH-CH2-CH3+HCl$��_{��}^{����}$ | |
| C�� | CH3-CH=CH2+H2O$��_{���ȡ���ѹ}^{����}$ | |
| D�� | CH4+Cl2$\stackrel{����}{��}$ |
